

68 Best Chemistry Experiments: Learn About Chemical Reactions
Whether you’re a student eager to explore the wonders of chemical reactions or a teacher seeking to inspire and engage your students, we’ve compiled a curated list of the top 68 chemistry experiments so you can learn about chemical reactions.
While the theories and laws governing chemistry can sometimes feel abstract, experiments bridge the gap between these concepts and their tangible manifestations. These experiments provide hands-on experiences illuminating the intricacies of chemical reactions, molecular structures, and elemental properties.
1. Covalent Bonds

By engaging in activities that demonstrate the formation and properties of covalent bonds, students can grasp the significance of these bonds in holding atoms together and shaping the world around us.
Learn more: Covalent Bonds
2. Sulfuric Acid and Sugar Demonstration
Through this experiment, students can develop a deeper understanding of chemical properties, appreciate the power of chemical reactions, and ignite their passion for scientific exploration.
3. Make Hot Ice at Home
Making hot ice at home is a fascinating chemistry experiment that allows students to witness the captivating transformation of a liquid into a solid with a surprising twist.
4. Make a Bouncing Polymer Ball

This hands-on activity not only allows students to explore the fascinating properties of polymers but also encourages experimentation and creativity.
Learn more: Thought Co
5. Diffusion Watercolor Art

This experiment offers a wonderful opportunity for students to explore the properties of pigments, observe how they interact with water, and discover the mesmerizing patterns and textures that emerge.
Learn more: Diffusion Watercolor Art
6. Exploding Baggie

The exploding baggie experiment is a captivating and dynamic demonstration that students should engage in with caution and under the supervision of a qualified instructor.
Learn more: Exploding Baggie
7. Color Changing Chemistry Clock

This experiment not only engages students in the world of chemical kinetics but also introduces them to the concept of a chemical clock, where the color change acts as a timekeeping mechanism.
Learn more: Color Changing Chemistry Clock
8. Pipe Cleaner Crystal Trees

By adjusting the concentration of the Borax solution or experimenting with different pipe cleaner arrangements, students can customize their crystal trees and observe how it affects the growth patterns.
Learn more: Pipe Cleaner Crystal Trees
9. How To Make Ice Sculptures

Through this experiment, students gain a deeper understanding of the physical and chemical changes that occur when water freezes and melts.
Learn more: Ice Sculpture
10. How to Make Paper

Through this hands-on activity, students gain a deeper understanding of the properties of cellulose fibers and the transformative power of chemical reactions.
Learn more: How to Make Paper
11. Color Changing Chemistry
Color changing chemistry is an enchanting experiment that offers a captivating blend of science and art. Students should embark on this colorful journey to witness the mesmerizing transformations of chemicals and explore the principles of chemical reactions.
12. Gassy Banana
The gassy banana experiment is a fun and interactive way for students to explore the principles of chemical reactions and gas production.
Learn more: Gassy Banana
13. Gingerbread Man Chemistry Experiment

This hands-on activity not only introduces students to the concepts of chemical leavening and heat-induced reactions but also allows for creativity in decorating and personalizing their gingerbread creations.
Learn more: Gingerbread Man Chemistry Experiment
14. Make Amortentia Potion

While the love potion is fictional, this activity offers a chance to explore the art of potion-making and the chemistry behind it.
Learn more: How to Make Amortentia Potion
15. Strawberry DNA Extraction
This hands-on experiment offers a unique opportunity to observe DNA, the building blocks of life, up close and learn about its structure and properties.
16. Melting Snowman

The melting snowman experiment is a fun and whimsical activity that allows students to explore the principles of heat transfer and phase changes.
Learn more: Melting Snowman
17. Acid Base Cabbage Juice

The acid-base cabbage juice experiment is an engaging and colorful activity that allows students to explore the pH scale and the properties of acids and bases.
By extracting the purple pigment from red cabbage leaves and creating cabbage juice, students can use this natural indicator to identify and differentiate between acidic and basic substances.
Learn more: Acid Base Cabbage Juice
18. Magic Milk

The magic milk experiment is a mesmerizing and educational activity that allows students to explore the concepts of surface tension and chemical reactions.
By adding drops of different food colors to a dish of milk and then introducing a small amount of dish soap, students can witness a captivating display of swirling colors and patterns.
Learn more: Magic Milk
19. Melting Ice with Salt and Water

Through this hands-on activity, students can gain a deeper understanding of the science behind de-icing and how different substances can influence the physical properties of water.
Learn more: Melting Ice with Salt and Water
20. Barking Dog Chemistry Demonstration

The barking dog chemistry demonstration is an exciting and visually captivating experiment that showcases the principles of combustion and gas production.
21. How to Make Egg Geodes

Making egg geodes is a fascinating and creative chemistry experiment that students should try. By using common materials like eggshells, salt, and food coloring, students can create their own beautiful geode-like crystals.
Learn more: How to Make Egg Geodes
22. Make Sherbet

This experiment not only engages the taste buds but also introduces concepts of acidity, solubility, and the chemical reactions that occur when the sherbet comes into contact with moisture.
Learn more: Make Sherbet
23. Hatch a Baking Soda Dinosaur Egg

As the baking soda dries and hardens around the toy, it forms a “shell” resembling a dinosaur egg. To hatch the egg, students can pour vinegar onto the shell, causing a chemical reaction that produces carbon dioxide gas.
Learn more: Steam Powered Family
24. Chromatography Flowers

By analyzing the resulting patterns, students can gain insights into the different pigments present in flowers and the science behind their colors.
Learn more: Chromatography Flowers
25. Turn Juice Into Solid

Turning juice into a solid through gelification is an engaging and educational chemistry experiment that students should try. By exploring the transformation of a liquid into a solid, students can gain insights of chemical reactions and molecular interactions.
Learn more: Turn Juice into Solid
26. Bouncy Balls
Making bouncy balls allows students to explore the fascinating properties of polymers, such as their ability to stretch and rebound.
27. Make a Lemon Battery
Creating a lemon battery is a captivating and hands-on experiment that allows students to explore the fundamentals of electricity and chemical reactions.
28. Mentos and Soda Project
The Mentos and soda project is a thrilling and explosive experiment that students should try. By dropping Mentos candies into a bottle of carbonated soda, an exciting eruption occurs.
29. Alkali Metal in Water
The reaction of alkali metals with water is a fascinating and visually captivating chemistry demonstration.
30. Rainbow Flame
The rainbow flame experiment is a captivating and visually stunning chemistry demonstration that students should explore.
31. Sugar Yeast Experiment
This experiment not only introduces students to the concept of fermentation but also allows them to witness the effects of a living organism, yeast, on the sugar substrate.
32. The Thermite Reaction
The thermite reaction is a highly energetic and visually striking chemical reaction that students can explore with caution and under proper supervision.
This experiment showcases the principles of exothermic reactions, oxidation-reduction, and the high temperatures that can be achieved through chemical reactions.
33. Polishing Pennies
Polishing pennies is a simple and enjoyable chemistry experiment that allows students to explore the concepts of oxidation and cleaning methods.
34. Elephant Toothpaste
The elephant toothpaste experiment is a thrilling and visually captivating chemistry demonstration that students should try with caution and under the guidance of a knowledgeable instructor.
35. Magic Potion
Creating a magic potion is an exciting and imaginative activity that allows students to explore their creativity while learning about the principles of chemistry.
36. Color Changing Acid-Base Experiment

Through the color changing acid-base experiment, students can gain a deeper understanding of chemical reactions and the role of pH in our daily lives.
Learn more: Color Changing Acid-Base Experiment
37. Fill up a Balloon
Filling up a balloon is a simple and enjoyable physics experiment that demonstrates the properties of air pressure. By blowing air into a balloon, you can observe how the balloon expands and becomes inflated.
38. Jello and Vinegar

The combination of Jello and vinegar is a fascinating and tasty chemistry experiment that demonstrates the effects of acid on a gelatin-based substance.
Learn more: Jello and Vinegar
39. Vinegar and Steel Wool Reaction

This experiment not only provides a visual demonstration of the oxidation process but also introduces students to the concept of corrosion and the role of acids in accelerating the process.
Learn more: Vinegar and Steel Wool Reaction
40. Dancing Rice

The dancing rice experiment is a captivating and educational demonstration that showcases the principles of density and buoyancy.
By pouring a small amount of uncooked rice into a clear container filled with water, students can witness the rice grains moving and “dancing” in the water.
Learn more: Dancing Rice
41. Soil Testing Garden Science

Soil testing is a valuable and informative experiment that allows students to assess the composition and properties of soil.
By collecting soil samples from different locations and analyzing them, students can gain insights into the nutrient content, pH level, and texture of the soil.
Learn more: Soil Testing Garden Science
42. Heat Sensitive Color Changing Slime

Creating heat-sensitive color-changing slime is a captivating and playful chemistry experiment that students should try.
Learn more: Left Brain Craft Brain
43. Experimenting with Viscosity

Experimenting with viscosity is an engaging and hands-on activity that allows students to explore the flow properties of liquids.
Viscosity refers to a liquid’s resistance to flow, and this experiment enables students to investigate how different factors affect viscosity.
Learn more: Experimenting with Viscosity
44. Rock Candy Science

Rock candy science is a delightful and educational chemistry experiment that students should try. By growing their own rock candy crystals, students can learn about crystal formation and explore the principles of solubility and saturation.
Learn more: Rock Candy Science
45. Baking Soda vs Baking Powder

Baking soda and baking powder have distinct properties that influence the leavening process in different ways.
This hands-on experiment provides a practical understanding of how these ingredients interact with acids and moisture to create carbon dioxide gas.
46. Endothermic and Exothermic Reactions Experiment

The endothermic and exothermic reactions experiment is an exciting and informative chemistry exploration that students should try.
By observing and comparing the heat changes in different reactions, students can gain a deeper understanding of energy transfer and the concepts of endothermic and exothermic processes.
Learn more: Education.com
47. Diaper Chemistry

By dissecting a diaper and examining its components, students can uncover the chemical processes that make diapers so effective at absorbing and retaining liquids.
Learn more: Diaper Chemistry
48. Candle Chemical Reaction
The “Flame out” experiment is an intriguing and educational chemistry demonstration that students should try. By exploring the effects of a chemical reaction on a burning candle, students can witness the captivating moment when the flame is extinguished.
49. Make Curds and Whey

This experiment not only introduces students to the concept of acid-base reactions but also offers an opportunity to explore the science behind cheese-making.
Learn more: Tinkerlab
50. Grow Crystals Overnight

By creating a supersaturated solution using substances like epsom salt, sugar, or borax, students can observe the fascinating process of crystal growth. This experiment allows students to explore the principles of solubility, saturation, and nucleation.
Learn more: Grow Crystals Overnight
51. Measure Electrolytes in Sports Drinks
The “Measure Electrolytes in Sports Drinks” experiment is an informative and practical chemistry activity that students should try.
By using simple tools like a multimeter or conductivity probe, students can measure the electrical conductivity of different sports drinks to determine their electrolyte content.
52. Oxygen and Fire Experiment
The oxygen and fire experiment is a captivating and educational chemistry demonstration that students should try. By observing the effects of oxygen on a controlled fire, students can witness the essential role of oxygen in supporting combustion.
53. Electrolysis Of Water
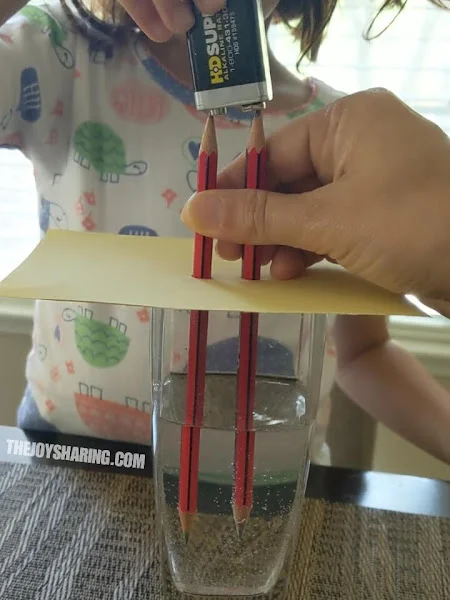
The electrolysis of water experiment is a captivating and educational chemistry demonstration that students should try.
Learn more: Electrolysis Of Water
54. Expanding Ivory Soap

The expanding Ivory Soap experiment is a fun and interactive chemistry activity that students should try. By placing a bar of Ivory soap in a microwave, students can witness the remarkable expansion of the soap as it heats up.
Learn more: Little Bins Little Hands
55. Glowing Fireworks

This experiment not only introduces students to the principles of pyrotechnics and combustion but also encourages observation, critical thinking, and an appreciation for the physics and chemistry behind.
Learn more: Glowing Fireworks
56. Colorful Polymer Chemistry

Colorful polymer chemistry is an exciting and vibrant experiment that students should try to explore polymers and colorants.
By combining different types of polymers with various colorants, such as food coloring or pigments, students can create a kaleidoscope of colors in their polymer creations.
Learn more: Colorful Polymer Chemistry
57. Sulfur Hexafluoride- Deep Voice Gas
This experiment provides a firsthand experience of how the density and composition of gases can influence sound transmission.
It encourages scientific curiosity, observation, and a sense of wonder as students witness the surprising transformation of their voices.
58. Liquid Nitrogen Ice Cream

Liquid nitrogen ice cream is a thrilling and delicious chemistry experiment that students should try. By combining cream, sugar, and flavorings with liquid nitrogen, students can create ice cream with a unique and creamy texture.
59. White Smoke Chemistry Demonstration

The White Smoke Chemistry Demonstration provides an engaging and visually captivating experience for students to explore chemical reactions and gases. By combining hydrochloric acid and ammonia solutions, students can witness the mesmerizing formation of white smoke.
60. Nitrogen Triiodide Chemistry Demonstration

The nitrogen triiodide chemistry demonstration is a remarkable and attention-grabbing experiment that students should try under the guidance of a knowledgeable instructor.
By reacting iodine crystals with concentrated ammonia, students can precipitate nitrogen triiodide (NI3), a highly sensitive compound.
61. Make a Plastic- Milk And Vinegar Reaction Experiment

Through the “Make a Plastic – Milk and Vinegar Reaction” experiment, students can gain a deeper understanding of the chemistry behind plastics, environmental sustainability, and the potential of biodegradable materials.
Learn more: Rookie Parenting
62. Eno and Water Experiment
This experiment not only introduces students to acid-base reactions but also engages their senses as they witness the visible and audible effects of the reaction.
63. The Eternal Kettle Experiment
By filling a kettle with alcohol and igniting it, students can investigate the behavior of the alcohol flame and its sustainability.
64. Coke and Chlorine Bombs
Engaging in this experiment allows students to experience the wonders of chemistry firsthand, making it an ideal choice to ignite their curiosity and passion for scientific exploration.
65. Set your Hand on Fire
This experiment showcases the fascinating nature of combustion and the science behind fire.
By carefully following proper procedures and safety guidelines, students can witness firsthand how the sanitizer’s high alcohol content interacts with an open flame, resulting in a brief but captivating display of controlled combustion.
66. Instant Ice Experiments
The Instant Ice Experiment offers an engaging and captivating opportunity for students to explore the wonders of chemistry and phase changes.
By using simple household ingredients, students can witness the fascinating phenomenon of rapid ice formation in just a matter of seconds.
67. Coke Cans in Acid and Base
Engaging in this experiment allows students to gain a deeper understanding of the chemical properties of substances and the importance of safety protocols in scientific investigations.
68. Color Changing Invisible Ink

The Color Changing Invisible Ink experiment offers an intriguing and fun opportunity for students to explore chemistry and learn about the concept of chemical reactions.
Learn more: Research Parent
Similar Posts:
- Top 100 Fine Motor Skills Activities for Toddlers and Preschoolers
- 37 Water Science Experiments: Fun & Easy
- Top 58 Creative Art Activities for Kids and Preschoolers
Leave a Comment Cancel reply
Save my name and email in this browser for the next time I comment.
Science Fun

Science Experiments for Kids:
Science experiments you can do at home! Explore an ever growing list of hundreds of fun and easy science experiments. Have fun trying these experiments at home or use them for science fair project ideas. Explore experiments by category, newest experiments, most popular experiments, easy at home experiments, or simply scroll down this page for tons of awesome experiment ideas!

Making A Volcano:
Acids and Bases Can Erupt in Your Faces

Orange Fizz:
Awesome Experiments:

New Experiments:
Check Out Our Newest Experiments

Top Experiments:

Easy Experiments:

Storm In A Glass:

Home Made Play Dough:

Snow Fluff:

Snow Globe:
Squishy Turkeys:

Rainbow in a Glass:

Sizzlin’ Snowballs:

Jello Lenses:

Ice Fishing:

Super Cool Soda:

Jack-O-Cano:

Dancing Hearts:

Marbled Gift Wrap:

Massive Expanding Soap:

Surface Tension Art:

Fizzy Fruit:
Rotting Pumpkin:

Explode A Bag:

Invisible Extinguisher:

Paper Hovercrafts:

Fun Fossil Stamps:

Cool Crystals:
Balloon Pop! Not!

Solar Eclipse Kit:

Moldy Apples:

Cool Off Volcanoes:

Vinegar Pops:

Make It Rain:

Black Light Blue Beverage:

Changing of the Leaves:

Snowflakes:
Water Fireworks:

Mind of a Student:

Balloon Speakers:

Polar Bear Blubber:
Gorgeous Gooey Gobstoppers:

Olympic Medals:

Dyed Flowers:

Rain, Rain, Don’t Go Away Gauge:

Blossoming Beans:
Butter Fingers:

Polishing Pennies:

Dancing Liquid:

Floating Egg:
Bendy Bones:

Pot Of Gold:

Layers of Liquids:

Crystal Candy:
- Random article
- Teaching guide
- Privacy & cookies

by Chris Woodford . Last updated: January 6, 2023.
Photo: There are always new theories to test and experiments to try. Even when we've completely nailed how Earth works, there's still the rest of the Universe to explore! Fourier telescope experiment photo by courtesy of NASA .
1: Galileo demonstrates that objects fall at the same speed (1589)
Photo: Galileo proved that different things fall at the same speed.
2: Isaac Newton splits white light into colors (1672)
Artwork: A glass prism splits white light into a spectrum. Nature recreates Newton's famous experiment whenever you see a rainbow!
3: Henry Cavendish weighs the world (1798)
Artwork: Henry Cavendish's experiment seen from above. 1) Two small balls, connected by a stick, are suspended by a thread so they're free to rotate. 2) The balls are attracted by two much larger (more massive) balls, fixed in place. 3) A light beam shines from the side at a mirror (green), mounted so it moves with the small balls. The beam is reflected back onto a measuring scale. 4) As the two sets of balls attract, the mirror pivots, shifting the reflected beam along the scale, so allowing the movement to be measured.
4: Thomas Young proves light is a wave... or does he? (1803)
Artwork: Thomas Young's famous double-slit experiment proved that light behaved like a wave—at least, some of the time. Left: A laser (1) produces coherent (regular, in-step) light (2) that passes through a pair of slits (3) onto a screen (4). If Newton were completely correct, we'd expect to see a single bright area on the screen and darkness either side. What we actually see is shown on the right. Light appears to ripple out in waves from the two slits (5), producing a distinctive interference pattern of light and dark areas (6).
5: James Prescott Joule demonstrates the conservation of energy (1840)
Artwork: The "Mechanical Equivalent of Heat"—James Prescott Joule's famous experiment proving the law now known as the conservation of energy.
6: Hippolyte Fizeau measures the speed of light (1851)
Artwork: How Fizeau measured the speed of light.
7: Robert Millikan measures the charge on the electron (1909)
Artwork: How Millikan measured the charge on the electron. 1) Oil drops (yellow) are squirted into the experimental apparatus, which has a large positive plate (blue) on top and a large negative plate (red) beneath. 2) X rays (green) are fired in. 3) The X rays give the oil drops a negative electrical charge. 4) The negatively charged drops can be made to "float" in between the two plates so their weight (red) is exactly balanced by the upward electrical pull of the positive plate (blue). When these two forces are equal, we can easily calculate the charge on the drops, which is always a whole number multiple of the basic charge on the electron.
8: Ernest Rutherford (and associates) split the atom (1897–1932)
Artwork: Transmutation: When Rutherford fired alpha particles (helium nuclei) at nitrogen, he produced oxygen. As he later wrote: "We must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle, and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus." In other words, he had split one atom apart to make another one.
Artwork: In Rutherford's gold-foil experiment (also known as the Geiger-Marsden experiment), atoms in a sheet of gold foil (1) allow positively charged alpha particles to pass through them (2) as long as the particles are traveling clear of the nucleus. Any particles fired at the nucleus are deflected by its positive charge (3). Fired at exactly the right angle, they will bounce right back! While this experiment is not splitting any atoms, as such, it was a key part of the decades-long effort to understand what atoms are made of—and in that sense, it did help physicists to "split" (venture inside) the atom.
9: Enrico Fermi demonstrates the nuclear chain reaction (1942)
Artwork: The nuclear chain reaction that turns uranium-235 into uranium-236 with a huge release of energy.
10: Rosalind Franklin photographs DNA with X rays (1953)
Artwork: The double-helix structure of DNA. Photographed with X rays, these intertwined curves appear as an X shape. Studying the X pattern in one of Franklin's photos was an important clue that tipped off Crick and Watson about the double helix.
If you liked this article...
Don't want to read our articles try listening instead, find out more, on this website.
- Six Easy Pieces by Richard Feynman. Basic Books, 2011. This book isn't half as "easy" as the title suggests, but it does contain interesting introductions to some of the topics covered here, including the conservation of energy, the double-slit experiment, and quantum theory.
- The Oxford Handbook of the History of Physics by Jed Z. Buchwald and Robert Fox (eds). Oxford University Press, 2013/2017. A collection of twenty nine scholarly essays charting the history of physics from Galileo's gravity to the age of silicon chips.
- Great Experiments in Physics: Firsthand Accounts from Galileo to Einstein Edited by Maurice Shamos. Dover, 1959/1987. This is one of my favorite science books, ever. It's a great compilation of some classic physics experiments (including four of those listed here—the experiments by Henry Cavendish, Thomas Young, James Joule, and Robert Millikan) written by the experimenters themselves. A rare opportunity to read firsthand accounts of first-rate science!
Text copyright © Chris Woodford 2012, 2023. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback
- Grades 6-12
- School Leaders
Have You Seen Our List of Favorite Graphic Novels?
Every product is independently selected by our team of teacher-reviewers and editors. Things you buy through our links may earn us a commission.
50 Sensational 7th Grade Science Fair Projects and Classroom Activities
Mummification, oxidation, electroplating, and more!

Engage every student with these 7th grade science fair projects, whether they’re interested in biology, chemistry, physics, environmental science, or any other discipline. Plus, find interesting classroom demos, experiments, and hands-on activities to spice up your lesson plans!
To make it easier to find classroom activities or science fair ideas for 7th grade, we’ve rated all the projects by difficulty and the materials needed:
Difficulty:
- Easy: Low or no-prep experiments you can do pretty much anytime
- Medium: These take a little more setup or a longer time to complete
- Advanced: Experiments like these take a fairly big commitment of time or effort
- Basic: Simple items you probably already have around the house
- Medium: Items that you might not already have but are easy to get your hands on
- Advanced: These require specialized or more expensive supplies to complete
Biology and Ecology Science Fair Ideas for 7th Grade
Chemistry science fair ideas for 7th grade, physics and engineering science fair ideas for 7th grade, 7th grade science classroom demos, experiments, and hands-on activities.
Want to learn more about animals or human behavior, the environment around you, or other life science topics? Try these 7th grade science fair projects.
Learn whether color affects memory

Difficulty: Easy / Materials: Medium
Can certain colors improve your memory? This experiment explores that idea using different text, background colors, and blue light settings on devices.
Learn more: Colors and Memory at Education.com
Explore how sugary drinks affect teeth

Difficulty: Easy / Materials: Medium ADVERTISEMENT
The calcium content of eggshells makes them a great stand-in for teeth. In this experiment, students use eggs to determine how soda and juice stain the teeth and wear down the enamel. (Bonus: Have students try different toothpaste and toothbrush combinations to see how effective they are.)
Learn more: Eggshell Experiment at Feels Like Home
Extract DNA from an onion
Difficulty: Medium / Materials: Medium
Learn how to extract DNA from an onion (most of what you need you can find at home, and you can get 95% ethanol at Amazon ). Then, turn it into an experiment by applying the theory to other fruits or vegetables to see if you can get similar results.
Stretch your mind with a flexibility experiment

Difficulty: Medium / Materials: Basic
Find out how important stretching really is by comparing the flexibility of willing test subjects before and after stretch exercises. This is a great experiment for fitness fans.
Learn more: Flexibility Experiment at We Have Kids
Construct a DIY grow box

Design a grow box using a cardboard box, foil, and a plug-in light socket . Then, use it for all kinds of plant-based science fair ideas for 7th grade students.
Learn more: DIY Grow Box at Uplifting Mayhem
Collect and control biofilm

Bacteria that accumulate on objects in the water form a substance called biofilm. In this 7th grade science fair project, students build an apparatus to collect biofilm and then experiment with ways to reduce the amount of biofilm that accumulates over time.
Learn more: Biofilm Experiment at The Homeschool Scientist
See if caffeine helps you type faster
People seek out a jolt of caffeine when they’re feeling sluggish, but does it really help them perform better? This 7th grade science fair project tasks students with answering that question using the scientific method.
Find out if all plants are phototropic
You probably already know that many plants grow toward the light. But do all of them respond in the same way? Test several types of plants to find out.
Devise a water filtration system

Plenty of homes use water filtration systems these days, but how do they really work? This chemistry experiment explores how charcoal filters impurities from drinking water.
Learn more: Water Filtration at The Homeschool Scientist
Determine whether text abbreviations are a new language

Kids are fluent in text-speak, but does it really count as a whole new language? In this 7th grade science fair project, students research language and the history of texting, then compile a texting glossary and consider texting’s practical applications.
Learn more: Text Language at Education.com
If you’re fascinated by test tubes, beakers, and Bunsen burners, check out these interesting 7th grade science fair projects and ideas.
Design your own slime
Chances are good your students already love making and playing with slime. Turn the fun into an experiment by changing the ingredients to create slime with a variety of properties, from magnetic to glow-in-the-dark!
Copper-plate some coins

Students need just a few simple supplies to perform electroplating, but the results are always impressive. (Get copper strips and 9V battery snap connectors with alligator clips on Amazon.) Turn this into a 7th grade science fair project by changing up the variables (does temperature matter?) or items being electroplated.
Learn more: Electroplating at KiwiCo
Swab and test for germs

Germ experiments are one of the most popular science fair ideas for 7th grade students. Swab household items, school supplies, and more to discover what’s living on the items around you.
Learn more: Germ Experiment at Angelic Scalliwags
Spherify your favorite beverage
Spherification is a hot trend in top restaurants, but 7th grade science students can easily replicate it at home with a spherification kit . This is a cool chemistry experiment, and tasty too!
Test calorie counts in packaged foods
Ever wonder how scientists determine how many calories are in your food? Try this experiment to find out!
Explore mummification
First, learn how to mummify a hot dog using baking soda as a desiccant. Then, experiment with other desiccants or items to turn this into a bona fide experiment.
Play around with oxidation
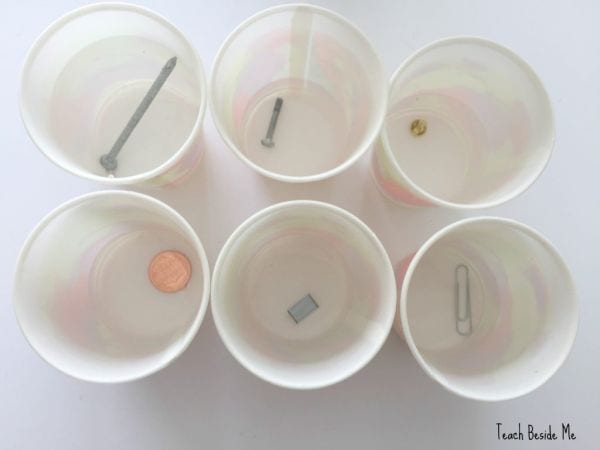
Can you find a way to slow or prevent oxidation (rusting)? This is one of those 7th grade science fair ideas that’s simple in concept but has lots of practical applications.
Learn more: Oxidation Experiment at Teach Beside Me
Blow hot or cold bubbles

Blowing bubbles may sound like too much fun for a science project, but when conditions like temperature are altered, the experimental part kicks in. What conditions do you need to blow a bubble that freezes?
Learn more: Bubble Life & Temperature at ThoughtCo.
Whip up some eggshell chalk

Use the calcium in eggshells to make your own sidewalk chalk. Then, tinker with the recipe to see if you can make the chalk last longer, resist water, or other variables.
Learn more: DIY Chalk at Kidspot
See the effect of acid rain on plants

Difficulty: Easy / Materials: Basic
This simple project tests whether acid rain has any effect on plant life, using vinegar in place of fossil fuels. Experiment with different acid concentrations and pH levels for a more advanced version.
Learn more: Acid Rain Experiment at STEAM Powered Family
Explore the laws of motion, the science of energy, or STEM challenge engineering ideas through 7th grade science fair projects like these.
Drive a balloon-powered car

Engineer a balloon-powered car using basic materials from around the house (even the wheels are bottle caps!). Experiment to see how far or fast you can make the car go.
Learn more: Balloon Car at Prolab
Construct a geodesic dome
Budding engineers will love designing, building, and testing the strength of the fascinating geodesic dome. This experiment requires nothing more than newspaper and masking tape!
Design a solar oven

Students experiment with the best way to build a solar oven, exploring thermal energy, reflection, convection, and other physics concepts. They can serve up their experiment results along with their final reports!
Learn more: Solar Oven at Children’s Science Center
Lend a helping hand
This is a great individual or group 7th grade science project, as it encourages students to use and hone their design and engineering skills to make a working model of a hand. If you’ve got robotics skills, take this project to a more advanced level.
Build a Da Vinci bridge
There are plenty of bridge-building experiments out there, but this one is unique. It’s inspired by Leonardo da Vinci’s 500-year-old self-supporting wooden bridge. Build a model and test the amount of weight it can hold, or craft a full-size version to put Da Vinci’s plan into action.
Construct a water clock

You’ll blow your 7th grade science students’ minds when you tell them they’re going to build a clock using engineering that’s been around for thousands of years. The supplies are simple, but the results are pretty neat!
Learn more: Water Clock at STEAM Powered Family
Generate electricity
In this science fair project, kids build a generator from scratch. Turn it into an experiment by varying the materials to see if you can increase the amount of energy it produces.
Test the elasticity of balloons
Explore whether heat and cold have an effect on elasticity using balloons. Try this with other materials too to expand the project. ( Find more balloon science here! )
Freeze water in an instant
Explore the concept of nucleation (the process of chain reactions) by turning water into ice in seconds! Make this a 7th grade science fair project by trying the same process with other liquids.
Auto-feed your pet
Difficulty: Advanced / Materials: Advanced
Can you build a device that feeds your pets automatically? Even better, can you make it inexpensive and easy for others to build too? This project has real practical applications.
Use these classroom activities to teach human biology, mechanical engineering, and more physics and chemistry concepts in engaging and exciting ways.
Use Oreos to teach mitosis

A 7th grade science activity that doubles as a sweet treat? Your students are going to love this activity using Oreo cookies and colorful sprinkles to make cellular mitosis models.
Learn more: Oreo Mitosis at Ballin With Balling
Twist pipe cleaners to explore meiosis
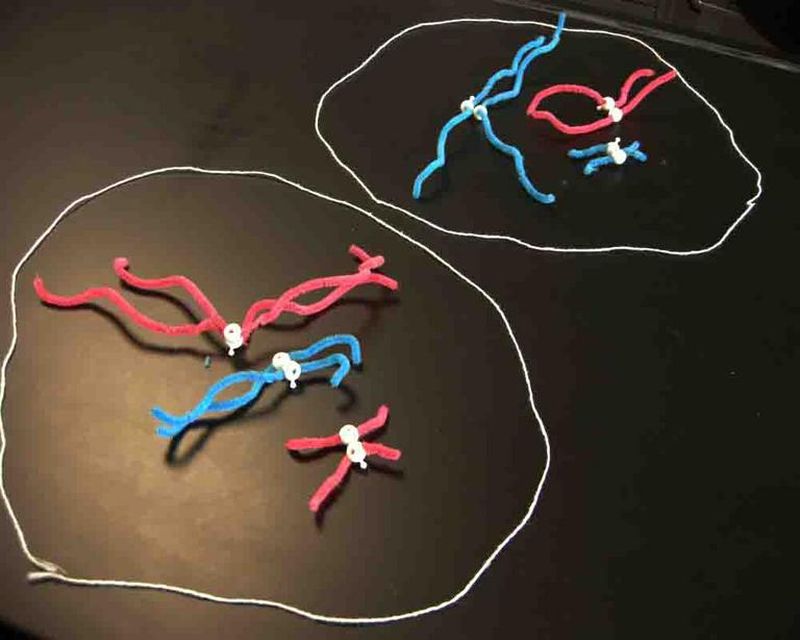
Meiosis is similar to mitosis, but it’s specific to the production of gametes. These hands-on models use basic materials like pipe cleaners and beads to make the process easier to visualize.
Learn more: Meiosis Models at Science Prof Online
Teach about “Homer-o-stasis”

Difficulty: Medium / Materials: Advanced
This is such a fun way to teach kids about the concept of homeostasis! Get all the instructions you need at the link.
Learn more: Homer-o-stasis at The Trendy Science Teacher
Sort jelly beans to learn genetics

If you’re learning about how genetic traits are passed along from parent to child, try this jelly-bean demo. When you’re finished, you can enjoy a sweet treat!
Learn more: Jelly Bean Genetics at The Owl Teacher
Design a pinball machine

Give your class basic supplies like rubber bands, plastic cups, and cardboard boxes. Then challenge them to create their very own pinball machines!
Learn more: Pinball STEM Challenge at Student Savvy
Conduct a carbon cycle lab activity

If you’ve got access to some basic chemicals, conduct this lab that helps students see the carbon cycle in action using their own breath.
Learn more: Science Lessons That Rock

Make a tea bag float on air

This easy experiment is a cool way to show kids how heat affects air molecules, making hot air rise. They’ll need some supervision with the fire, so try this out on the playground for extra safety.
Learn more: Floating Tea Bags at Coffee Cups and Crayons
Learn how salt affects density

Explore the salinity of various bodies of water, then re-create their waters to see if you can make an egg float or sink. Experiment with other objects too.
Learn more: Saltwater Density at Uplifting Mayhem
Watch the greenhouse effect in action

Climate change can be a contentious topic, so start by teaching kids about the greenhouse effect, which is easy to see and understand. Then, urge them to explore data collected by other scientists so they can learn to make informed decisions about topics like global warming.
Learn more: Greenhouse Effect at Teaching Science With Lynda
Blow bubbles to explore cell membranes

Kids are never too old to enjoy bubbles, so use them to learn more about cell membranes in this fun 7th grade science activity.
Learn more: Cell Membrane Bubbles at The Trendy Science Teacher
Marvel at a density rainbow
We learn early on that oil floats on water, but where do other liquids fit in? Students find out when they conduct this colorful density experiment that has them layer different substances, making a rainbow.
Ride the wave (machine)

Learning about wave action? Build this surprisingly easy wave machine for hands-on exploration.
Learn more: Wave Machine at Engaging Science Labs
Create a taxonomy system

Students can step into Linnaeus’ shoes by creating their own system of taxonomy using a handful of different dried beans. This is a fun 7th grade science project to do in groups, so students can see the differences between each group’s system.
Learn more: Taxonomy Project at Our Journey Westward
Bake an edible cell model

Sure, students could build a cell model out of clay, but cake and candy are so much more delicious! Check out the link below to see how one teacher does it.
Learn more: Edible Cell Model at Weird Unsocialized Homeschooling
Swing a glass of water
This classic science experiment teaches kids about centripetal force. Be forewarned: This could potentially make a bit of a mess, so consider taking this one outside.
Simulate natural selection with a lab activity

Travel to the Galápagos Islands and follow in Darwin’s footsteps as students explore finch beak adaptations in this clever natural selection lab.
Learn more: Natural Selection Lab at Teach To Serve
Participate in Project FeederWatch

Citizen science projects bring science to life for kids! One of our favorites is Project FeederWatch, where kids put out bird feeders and then count and report on their visitors. This is a great way to build a love of birding for life.
Learn more: Classroom Resources at Project FeederWatch
Experiment with basic substances to learn about chemical change

If you’re introducing lab work and chemistry basics to 7th graders, this easy lab is a great way to do it. They’ll learn safety procedures and get to feel like “real” scientists as they pour, mix, swirl, and more.
Learn more: Chemical Change Lab at Super Sass and Science Class
Assemble an edible DNA model

DNA models are always more fun when you can snack on them afterwards. Want to make this a healthier activity? Use fruits and veggies to make models instead.
Learn more: Edible DNA Model at Hess UnAcademy
Craft a food web marble maze

Combine a STEM challenge with learning about food webs in this clever project. Kids will love the hands-on aspect, and it will really help the learning stick.
Learn more: Food Web Marble Maze at Teach Savvy
Keep the STEM learning going with these 15 Items All Middle School Math Classrooms Need .
Plus if you like these 7th grade science fair projects, sign up for our newsletters and get all the latest teacher tips and ideas, straight to your inbox.

You Might Also Like

The Big List of Science Fair Project Ideas, Resources, and More
Options for every age, interest, and skill level! Continue Reading
Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
The Top Ten Scientific Discoveries of the Decade
Breakthroughs include measuring the true nature of the universe, finding new species of human ancestors, and unlocking new ways to fight disease
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/bennett.jpg)
Jay Bennett
Former associate web editor, science.
:focal(800x601:801x602)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/d4/a8/d4a88985-6b69-41f0-85b3-4425e6c98596/science_rules5.jpg)
Millions of new scientific research papers are published every year , shedding light on everything from the evolution of stars to the ongoing impacts of climate change to the health benefits (or determents) of coffee to the tendency of your cat to ignore you . With so much research coming out every year, it can be difficult to know what is significant, what is interesting but largely insignificant, and what is just plain bad science . But over the course of a decade, we can look back at some of the most important and awe-inspiring areas of research, often expressed in multiple findings and research papers that lead to a true proliferation of knowledge. Here are ten of the biggest strides made by scientists in the last ten years.
New Human Relatives
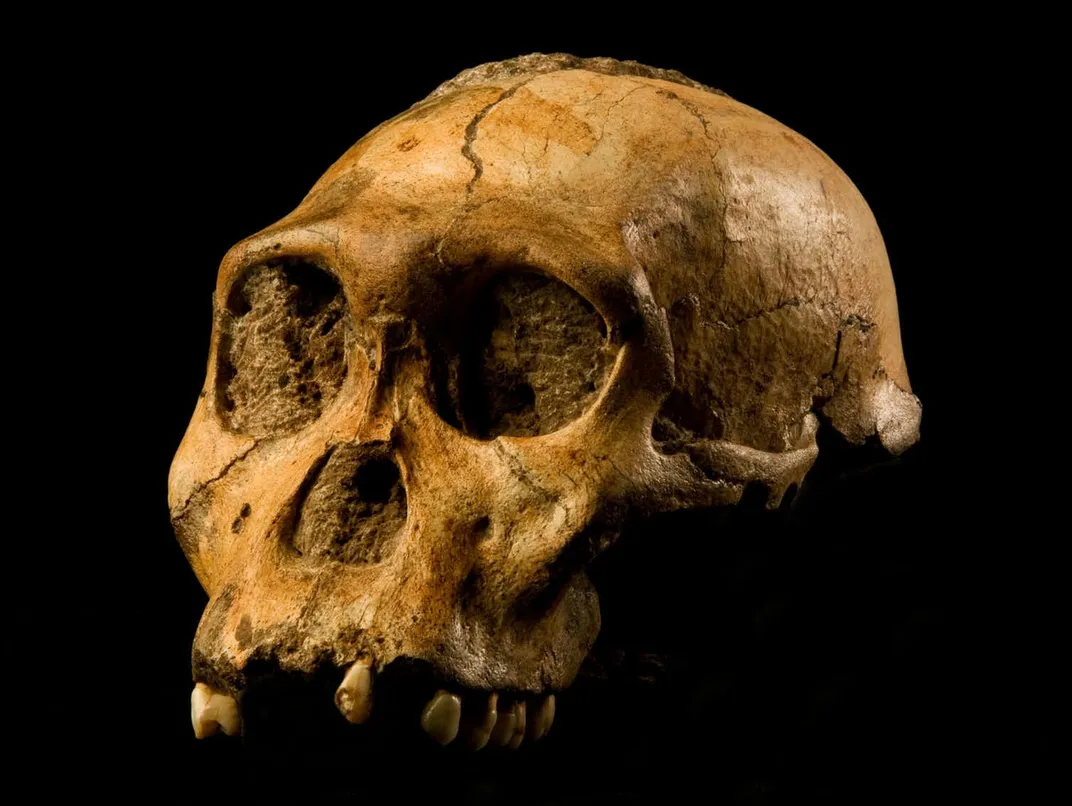
The human family tree expanded significantly in the past decade, with fossils of new hominin species discovered in Africa and the Philippines. The decade began with the discovery and identification of Australopithecus sediba , a hominin species that lived nearly two million years ago in present-day South Africa. Matthew Berger, the son of paleoanthropologist Lee Berger, stumbled upon the first fossil of the species, a right clavicle, in 2008, when he was only 9 years old. A team then unearthed more fossils from the individual, a young boy, including a well-preserved skull, and A. sediba was described by Lee Berger and colleagues in 2010 . The species represents a transitionary phase between the genus Australopithecus and the genus Homo , with some traits of the older primate group but a style of walking that resembled modern humans.
Also discovered in South Africa by a team led by Berger, Homo naledi lived much more recently, some 335,000 to 236,000 years ago, meaning it may have overlapped with our own species, Homo sapiens. The species, first discovered in the Rising Star Cave system in 2013 and described in 2015 , also had a mix of primitive and modern features, such as a small brain case (about one-third the size of Homo sapiens ) and a large body for the time, weighing approximately 100 pounds and standing up to five feet tall. The smaller Homo luzonensis (three to four feet tall) lived in the Philippines some 50,000 to 67,000 years ago , overlapping with several species of hominin. The first H. luzonensis fossils were originally identified as Homo sapiens, but a 2019 analysis determined that the bones belonged to an entirely unknown species.
These three major finds in the last ten years suggest that the bones of more species of ancient human relatives are likely hidden in the caves and sediment deposits of the world, waiting to be discovered.
Taking Measure of the Cosmos

When Albert Einstein first published the general theory of relativity in 1915, he likely couldn’t have imagined that 100 years later, astronomers would test the theory’s predictions with some of the most sophisticated instruments ever built—and the theory would pass each test. General relativity describes the universe as a “fabric” of space-time that is warped by large masses. It’s this warping that causes gravity, rather than an internal property of mass as Isaac Newton thought.
One prediction of this model is that the acceleration of masses can cause “ripples” in space-time, or the propagation of gravitational waves. With a large enough mass, such as a black hole or a neutron star, these ripples may even be detected by astronomers on Earth. In September 2015, the LIGO and Virgo collaboration detected gravitational waves for the first time, propagating from a pair of merging black holes some 1.3 billion light-years away. Since then, the two instruments have detected several additional gravitational waves , including one from a two merging neutron stars.
Another prediction of general relativity—one that Einstein himself famously doubted —is the existence of black holes at all, or points of gravitational collapse in space with infinite density and infinitesimal volume. These objects consume all matter and light that strays too close, creating a disk of superheated material falling into the black hole. In 2017, the Event Horizon Telescope collaboration —a network of linked radio telescopes around the world—took observations that would later result in the first image of the environment around a black hole, released in April 2019 .
The Hottest Years on Record

Scientists have been predicating the effects of burning coal and fossil fuels on the temperature of the planet for over 100 years. A 1912 issue of Popular Mechanics contains an article titled “ Remarkable Weather of 1911: The Effect of the Combustion of Coal on the Climate—What Scientists Predict for the Future ,” which has a caption that reads: “The furnaces of the world are now burning about 2,000,000,000 tons of coal a year. When this is burned, uniting with oxygen, it adds about 7,000,000,000 tons of carbon dioxide to the atmosphere yearly. This tends to make the air a more effective blanket for the earth and to raise its temperature. The effect may be considerable in a few centuries.”
Just one century later, and the effect is considerable indeed. Increased greenhouse gases in the atmosphere have produced hotter global temperatures, with the last five years (2014 to 2018) being the hottest years on record . 2016 was the hottest year since the National Oceanic and Atmospheric Administration (NOAA) started recording global temperature 139 years ago. The effects of this global change include more frequent and destructive wildfires, more common droughts, accelerating polar ice melt and increased storm surges. California is burning, Venice is flooding, urban heat deaths are on the rise, and countless coastal and island communities face an existential crisis—not to mention the ecological havoc wreaked by climate change, stifling the planet’s ability to pull carbon back out of the atmosphere.
In 2015, the United Nations Framework Convention on Climate Change (UNFCCC) reached a consensus on climate action, known as the Paris Agreement. The primary goal of the Paris Agreement is to limit global temperature increases to 1.5 degrees Celsius over pre-industrial levels . To achieve this goal, major societal transformations will be required, including replacing fossil fuels with clean energy such as wind, solar and nuclear; reforming agricultural practices to limit emissions and protect forested areas; and perhaps even building artificial means of pulling carbon dioxide out of the atmosphere.
Editing Genes

Ever since the double-helix structure of DNA was revealed in the early 1950s , scientists have hypothesized about the possibility of artificially modifying DNA to change the functions of an organism. The first approved gene therapy trial occurred in 1990, when a four-year-old girl had her own white blood cells removed, augmented with the genes that produce an enzyme called adenosine deaminase (ADA), and then reinjected into her body to treat ADA deficiency, a genetic condition that hampers the immune system’s ability to fight disease. The patient’s body began producing the ADA enzyme, but new white blood cells with the corrected gene were not produced, and she had to continue receiving injections .
Now, genetic engineering is more precise and available than ever before, thanks in large part to a new tool first used to modify eukaryotic cells (complex cells with a nucleus) in 2013 : CRISPR-Cas9. The gene editing tool works by locating a targeted section of DNA and “cutting” out that section with the Cas9 enzyme. An optional third step involves replacing the deleted section of DNA with new genetic material. The technique can be used for a wide range of applications, from increasing the muscle mass of livestock, to producing resistant and fruitful crops, to treating diseases like cancer by removing a patient’s immune system cells, modifying them to better fight a disease, and reinjecting them into the patient’s body.
In late 2018, Chinese researchers led by He Jiankui announced that they had used CRISPR-Cas9 to genetically modify human embryos, which were then transferred to a woman’s uterus and resulted in the birth of twin girls—the first gene-edited babies. The twins’ genomes were modified to make the girls more resistant to HIV, although the genetic alterations may have also resulted in unintended changes . The work was widely condemned by the scientific community as unethical and dangerous, revealing a need for stricter regulations for how these powerful new tools are used, particularly when it comes to changing the DNA of embryos and using those embryos to birth live children.
Mysteries of Other Worlds Revealed

Spacecraft and telescopes have revealed a wealth of information about worlds beyond our own in the last decade. In 2015, the New Horizons probe made a close pass of Pluto, taking the first nearby observations of the dwarf planet and its moons. The spacecraft revealed a surprisingly dynamic and active world, with icy mountains reaching up to nearly 20,000 feet and shifting plains that are no more than 10 million years old—meaning the geology is constantly changing. The fact that Pluto—which is an average of 3.7 billion miles from the sun , about 40 times the distance of Earth—is so geologically active suggests that even cold, distant worlds could get enough energy to heat their interiors, possibly harboring subsurface liquid water or even life.
A bit closer to home, the Cassini spacecraft orbited Saturn for 13 years , ending its mission in September 2017 when NASA intentionally plunged the spacecraft into the atmosphere of Saturn so it would burn up rather than continue orbiting the planet once it had exhausted its fuel. During its mission, Cassini discovered the processes that feed Saturn’s rings , observed a global storm encircle the gas giant, mapped the large moon Titan and found some of the ingredients for life in the plumes of icy material erupting from the watery moon Enceladus. In 2016, a year before the end of the Cassini mission, the Juno spacecraft arrived at Jupiter, where it has been measuring the magnetic field and atmospheric dynamics of the largest planet in the solar system to help scientists understand how Jupiter—and everything else around the sun—originally formed.
In 2012, the Curiosity rover landed on Mars, where it has made several significant discoveries, including new evidence of past water on the red planet , the presence of organic molecules that could be related to life, and mysterious seasonal cycles of methane and oxygen that hint at a dynamic world beneath the surface. In 2018, the European Space Agency announced that ground-penetrating radar data from the Mars Express spacecraft provided strong evidence that a liquid reservoir of water exists underground near the Martian south pole .
Meanwhile, two space telescopes, Kepler and TESS, have discovered thousands of planets orbiting other stars. Kepler launched in 2009 and ended its mission in 2018, revealing mysterious and distant planets by measuring the decrease in light when they pass in front of their stars. These planets include hot Jupiters, which orbit close to their stars in just days or hours; mini Neptunes, which are between the size of Earth and Neptune and may be gas, liquid, solid or some combination; and super Earths, which are large rocky planets that astronomers hope to study for signs of life. TESS, which launched in 2018, continues the search as Kepler’s successor. The space telescope has already discovered hundreds of worlds , and it could find 10,000 or even 20,000 before the end of the mission.
Fossilized Pigments Reveal the Colors of Dinosaurs

The decade began with a revolution in paleontology as scientists got their first look at the true colors of dinosaurs. First, in January 2010, an analysis of melanosomes—organelles that contain pigments—in the fossilized feathers of Sinosauropteryx , a dinosaur that lived in China some 120 to 125 million years ago, revealed that the prehistoric creature had “reddish-brown tones” and stripes along its tail . Shortly after, a full-body reconstruction revealed the colors of a small feathered dinosaur that lived some 160 million years ago , Anchiornis , which had black and white feathers on its body and a striking plume of red feathers on its head.
The study of fossilized pigments has continued to expose new information about prehistoric life, hinting at potential animal survival strategies by showing evidence of countershading and camouflage . In 2017, a remarkably well-preserved armored dinosaur which lived about 110 million years ago, Borealopelta , was found to have reddish-brown tones to help blend into the environment . This new ability to identify and study the colors of dinosaurs will continue to play an important role in paleontological research as scientists study the evolution of past life.
Redefining the Fundamental Unit of Mass
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/4d/4f/4d4f416e-6e5d-41fe-b6f7-3a79044a0aab/wattbalance_rsi_cover-1.jpg)
In November 2018, measurement scientists around the world voted to officially changed the definition of a kilogram , the fundamental unit of mass. Rather than basing the kilogram off of an object—a platinum-iridium alloy cylinder about the size of a golf ball—the new definition uses a constant of nature to set the unit of mass. The change replaced the last physical artifact used to define a unit of measure. (The meter bar was replaced in 1960 by a specific number of wavelengths of radiation from krypton, for example, and later updated to define a meter according to the distance light travels in a tiny fraction of a second .)
By using a sophisticated weighing machine known as a Kibble balance, scientists were able to precisely measure a kilogram according to the electromagnetic force required to hold it up. This electric measurement could then be expressed in terms of Planck’s constant, a number originally used by Max Planck to calculate bundles of energy coming from stars .
The kilogram was not the only unit of measure that was recently redefined. The changes to the International System of Units, which officially went into effect in May 2019 , also changed the definition for the ampere, the standard unit of electric current; the kelvin unit of temperature; and the mole, a unit of amount of substance used in chemistry. The changes to the kilogram and other units will allow more precise measurements for small amounts of material, such as pharmaceuticals, as well as give scientists around the world access to the fundamental units, rather than defining them according to objects that must be replicated and calibrated by a small number of labs.
First Ancient Human Genome Sequenced

In 2010, scientists gained a new tool to study the ancient past and the people who inhabited it. Researchers used a hair preserved in permafrost to sequence the genome of a man who lived some 4,000 years ago in what is now Greenland , revealing the physical traits and even the blood type of a member of one of the first cultures to settle in that part of the world. The first nearly complete reconstruction of a genome from ancient DNA opened the door for anthropologists and geneticists to learn more about the cultures of the distant past than ever before.
Extracting ancient DNA is a major challenge. Even if genetic material such as hair or skin is preserved, it is often contaminated with the DNA of microbes from the environment, so sophisticated sequencing techniques must be used to isolate the ancient human’s DNA. More recently, scientists have used the petrous bone of the skull , a highly dense bone near the ear, to extract ancient DNA.
Thousands of ancient human genomes have been sequenced since the first success in 2010, revealing new details about the rise and fall of lost civilizations and the migrations of people around the globe . Studying ancient genomes has identified multiple waves of migration back and forth across the frozen Bering land bridge between Siberia and Alaska between 5,000 and 15,000 years ago. Recently, the genome of a young girl in modern Denmark was sequenced from a 5,700-year-old piece of birch tar used as chewing gum, which also contained her mouth microbes and bits of food from one of her last meals.
A Vaccine and New Treatments to Fight Ebola
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/94/69/946983e3-aa54-4eeb-add1-1eea8092071c/gettyimages-1183891526.jpg)
This decade included the worst outbreak of Ebola virus diseases in history. The epidemic is believed to have begun with a single case of an 18-month-old-boy in Guinea infected by bats in December 2013. The disease quickly spread to neighboring countries, reaching the capitals of Liberia and Sierra Leone by July 2014, providing an unprecedented opportunity for the transmission of the disease to a large number of people. Ebola virus compromises the immune system and can cause massive hemorrhaging and multiple organ failure. Two and a half years after the initial case, more than 28,600 people had been infected, resulting in at least 11,325 deaths, according to the CDC .
The epidemic prompted health officials to redouble their efforts to find an effective vaccine to fight Ebola. A vaccine known as Ervebo, made by the pharmaceutical company Merck, was tested in a clinical trial in Guinea performed toward the end of the outbreak in 2016 that proved the vaccine effective. Another Ebola outbreak was declared in the Democratic Republic of the Congo in August 2018, and the ongoing epidemic has spread to become the deadliest since the West Africa outbreak, with 3,366 reported cases and 2,227 deaths as of December 2019 . Ervebo has been used in the DRC to fight the outbreak on an expanded access or “compassionate use” basis . In November 2019, Ervebo was approved by the European Medicines Agency (EMA) , and a month later it was approved in the U.S. by the FDA .
In addition to a preventative vaccine, researchers have been seeking a cure for Ebola in patients who have already been infected by the disease. Two treatments, which involve a one-time delivery of antibodies to prevent Ebola from infecting a patient’s cells, have recently shown promise in a clinical trial in the DRC . With a combination of vaccines and therapeutic treatments, healthcare officials hope to one day eradicate the viral infection for good .
CERN Detects the Higgs Boson
Over the past several decades, physicists have worked tirelessly to model the workings of the universe, developing what is known as the Standard Model. This model describes four basic interactions of matter, known as the fundamental forces . Two are familiar in everyday life: the gravitational force and the electromagnetic force. The other two, however, only exert their influence inside the nuclei of atoms: the strong nuclear force and the weak nuclear force.
Part of the Standard Model says that there is a universal quantum field that interacts with particles, giving them their masses. In the 1960s, theoretical physicists including François Englert and Peter Higgs described this field and its role in the Standard Model. It became known as the Higgs field, and according to the laws of quantum mechanics, all such fundamental fields should have an associated particle, which came to be known as the Higgs boson.
Decades later, in 2012, two teams using the Large Hadron Collider at CERN to conduct particle collisions reported the detection of a particle with the predicted mass of the Higgs boson, providing substantial evidence for the existence of the Higgs field and Higgs boson. In 2013, the Nobel Prize in Physics was awarded to Englert and Higgs “for the theoretical discovery of a mechanism that contributes to our understanding of the origin of mass of subatomic particles, and which recently was confirmed through the discovery of the predicted fundamental particle.” As physicists continue to refine the Standard Model, the function and discovery of the Higgs boson will remain a fundamental part of how all matter gets its mass, and therefore, how any matter exists at all.
Get the latest Science stories in your inbox.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/bennett.jpg)
Jay Bennett | | READ MORE
Jay Bennett was the associate web editor, science, for Smithsonian .

32 Cool Science Experiments for Kids (that are Fun AND Easy!)

Do you ever want to do science experiments at home with your kids, but you’re not quite sure what to do? Not just any old kitchen science experiment will do – you want something cooler than vinegar + bicarb soda! But, you also want something simple and easy to do – because no-one wants a huge mess from their kids doing crazy science experiments at home!
We understand, and that’s why the writing team here at STEM Geek has put our heads together to come up with the most awesome at-home science experiments for kids! As science enthusiasts and educators, we also wanted to make sure that these are genuine science learning opportunities. So not only are they captivating for the kids, but we also emphasize what questions can be asked as kids explore and apply the scientific method! Plus, we’ve arranged them according to how much time they take: up to 1 hour, 1 to several hours, and long-term.
Related Post: Ultimate Boredom Buster: 101 Things To Do When Kids Are Bored
Science Experiments at Home that take Less than 1 Hour
1. tie-dye milk.
Sounds delicious, right? You’re not actually drinking it, but instead watching science magic happens when you combine dish soap with milk and food coloring. This is a very pretty experiment that draws the focus and mind into what’s happening on the plate, and all because of a little chemistry with everyday items. Well, food dye may not be an everyday item, but it might be after your kids get a hold of this!
So, what’s going on here, scientifically-speaking? Milk is made up of two major ingredients: water and fat. When you add a little dish soap, it bonds with the fat in the milk so strongly that it literally pushes the food coloring and water away from the cotton ball. On a microscopic level, the dish soap is wandering around the milk, which causes the colors to swirl and swirl.
Questions to ask beforehand:
- Before knowing what will happen to the food coloring, ask the kids what they think will happen when dish soap mixes with milk.
- Since the major catalyst is fat in the milk, what would happen if you used other types of milk: Skim milk, soy milk, coconut milk?
You’ll need:
- Round cake pan or plate with high edges
- Cotton ball (some tutorials show cotton swabs)
- Dish detergent
- Different colors of food dye (three or four should do)
Procedure/Instructions:
- Fill the pan halfway with milk.
- Drip one color of food dye in one section of the plate away from the center. Four to five drops works and later you can play around with more or less. Do the same for the rest of the colors around the plate.
- Soak the cotton ball in dish detergent, and when you’re ready for action, place the cotton ball into the center of the pan.
- Watch the colors racing around, creating a psychedelic tie-dye effect!
- You can add more cotton balls throughout the dish to see more action.
- If some food coloring hugs the wall of the plate, take a cotton swab dipped in dish detergent and place it into the food coloring. It will move away!
2. Saturn’s Glowing Rings
I don’t know about you, but I love everything about space. This experiment shows you how Saturn’s rings are made of rocks and ice chunks even though they look so smooth in pictures. You’ll also see why there are big gaps in the rings. Younger kids take delight in using a flashlight and sprinkling powder, while older kids can get more specific with questions about Saturn and how the rocks and ice stay in orbit.
- Do Saturn’s rings give off their own light?
- Why are some rocks and ice chunks more lit up than others?
- Compare the results of light sprinkles to thicker sprinkles.
- Strong flashlight
- Powder (flour, baby powder, etc) in a shaker
- Very dark room
- Darken a room and set the flashlight on the edge of a table or counter, pointing it at a blank wall. Lay the newspaper on the floor between the flashlight and the wall.
- Turn on the flashlight and notice where the light comes from the flashlight and where it hits the wall. You should only see the light from these two places and not from the space between them. This shows you that the light travels through the air without being seen until it hits the wall. The light represents the sun’s light.
- Now to see how Saturn’s rings glow: Hold the powder shaker and sprinkle some powder over the beam of light where you know the light is traveling. You’ll notice the powder lights up and sparkles in the beam of light. The powder shows in glowing clumps, just like in Saturn’s rings.
3. Breaking Down Colors
We all know that the fun, vibrant colors we see in our lives are created by mixing the basic red, yellow, and blue. In this experiment, you and your child will learn which colors make up those fun shades they have in their art supplies. This also teaches some basic chemistry and uses materials you already have at home. It can be done very simply and expanded to create a large-scale investigation if you love it.
- Which colors separate out first?
- Is the same order for each test?
- Which colors make up the original shade?
- Do the different types of color (pen, pencil, paint) separate in the same way or differently?
- Are some separated in a shorter space are the colors the same mixture?
- Coffee filters
- Color sources (markers, colored pencils, paint, etc.)
- A plain pencil
- To complete this experiment, cut the coffee filters into strips, mark one end with a line the same distance from the bottom on each strip.
- Color in each strip (between the bottom and line) with your colors, and write at the top what the color and source are (e.g., purple marker).
- Place each strip in a glass and help it to stand up by folding the top over a pencil (a chopstick, table knife, or any long narrow object will also work) so that it stands up in the glass.
- Fill the glass up to the top of your colored block, and wait. The water will move up the filter, and the colors will separate out as it goes.
- Remove the strip once the water gets near the top of the strip to stop the experiment.
To make this a true experiment, we recommend testing multiple colors and using markers, colored pencils, and paint (as some starting examples). You could test the same colors from each type of art supply to investigate whether they all use the same mix of basic colors to create the same end product.
This post has a nice full description of the methods if you need more detail.
4. Water Xylophone

This simple experiment will teach your child about sound and pitch using glasses, water, and something to act as a mallet. Don’t let the simplicity deceive you, there are a lot of ways to experiment and learn through this process, and it also brings in an element of music that makes it interesting and engaging.
- Do you think more water makes the sound higher or lower in pitch?
- How do you think the shape or size of the glass will affect the sound?
- How should we arrange the glasses to play a simple song?
- Do you think this will work with a plastic cup, why or why not?
- Some glasses
- Something wood to act as a mallet (we recommend wood so you don’t break the glasses!)
- A great way to start is with glasses that are the same size, shape, and material, and filling them with different amounts of water.
- Have your child use the mallet to test how the amount of water affects the sound.
- From there, it’s a really simple extension to use different sized and shaped glasses (or any glass vessel like jars and bowls) to experiment with how the shape, size, and amount of water in the glass affect the tone.
To take this one further and really bring in the musical component, you and your child could work out a simple song and create the right tones to play it. If you or your child are musical, you could get very elaborate and creative (try googling harry potter or star wars theme songs on glasses, there are so many options that I couldn’t even choose one)!
5. Ultimate Bottle Flipping
Ah, bottle flipping. The fad that kids can’t get enough of, but parents are well and truly over. The constant thud of semi-filled water bottles being tossed (and hopefully landing upright) is guaranteed to send parents around the twist!
If you can stand it for a bit longer though, there’s a lot of STEM knowledge to be gained in this bottle flipping experiment. As we know, the aim of bottle flipping is to flip a partially filled water bottle underhand and get it to land upright.
In this experiment, kids will learn the importance of observing a result multiple times before changing a variable (the amount of water in the bottle).
- How much water should you put in the bottle?
- What is the ideal amount of liquid to get the perfect flip?
- What should be the ideal amount of water?
- Was their prediction correct?
- Why do they think the amount of water affects the chances of landing the bottle?
- A plastic water bottle
- Measuring jug
- Paper to record results
- Get the kids to start by flipping their bottle with no water in it at all. Kidspot recommends flipping it 50 times for each step, but you could do less if you need to.
- Try it again with 50ml of water.
- Keep adding more water until the bottle is full.
If they’re keen, you could try other types or sizes of bottles, or even try different liquids to see if that affects the results!
6. Rainbow in a Jar

This simple science experiment is not only very visually appealing, but it’s also a great way to learn about the density of liquids. Warning though, this one could get messy so make sure kids are in some old clothes and you might want to take it outside! I like this experiment because you’ll probably have most of the materials in your kitchen already!
- Which liquids they think will be heaviest?
- Which ones will be lightest?
- Why do they think that?
- A glass jar
- Food coloring
- Various liquids like honey, corn syrup, dishwashing liquid, olive oil, rubbing alcohol and water.
- Use the food coloring to make all your liquids a different color. A dropper comes in handy here, but if you don’t have one you can manage without.
- Slowly add each liquid to the jar (pouring into the middle of the jar is best).
- Soon, you’ll have different layers of colored liquid forming your very own rainbow in a jar.
You might even get them to draw a diagram of what they think the jar will look like at the end. They can compare this with the experiment results to see if their prediction was correct.
It might also help to talk to your kids first about what density is and how materials are all made of different amounts of molecules. The more molecules a liquid has, the heavier it will be. Playdough to Plato demonstrates a great way of introducing this concept using marbles.
7. Write Your Own Secret Messages!
We love science experiments that are made up primarily of supplies that you likely already have in your home.
- Why do you think this will work?
- Which liquid do you think will make the best secret message?
- Why do people write secret messages?
- Juice (eg. Lemon)
- Lamp (or anything else that can be used as a heat source)
- In order to complete this experiment, you’ll need to gather all of your supplies along with a piece of paper, some q-tips, and a lamp or other item that you can use as a heat source.
- Next, you’ll mix your lemon juice with a slight amount of water.
- Using your q-tip, use the mixture you’ve created to begin writing your message.
- Allow it to dry.
- Once dry, apply heat to it in order to get your message to appear.
Extend this project by attempting to write with a juice and water mixture, a milk and water mixture, or any other variation of the liquids we listed as necessary supplies!
8. Create Your Own Butterfly

Your little ones will love practicing their color mixing by creating their very own coffee filter butterflies. Hang them in the windows of your home to spread some cheer and to watch the sun flow through their beautiful wings!
- What colors can mix together to make other colors?
- How do butterflies fly?
- What do you think will happen when we add water to the markers?
- Water spray bottle
- Allow your child to draw on the coffee filter to their heart’s content.
- Spray it with water and allow the colors to mix together.
- Allow it to dry thoroughly.
- Once dry, fold it like a fan and then clip it in the middle.
Ta-da, you’ve created a beautiful butterfly!
9. Make A Duck Call
Give your family an excuse to head outdoors by allowing your children to craft their own duck calls. Test them out at a local pond and see if you can get the ducks to come closer to you for a healthy veggie snack!
- Do you think ducks will be able to hear us with this?
- What other materials do you think could make this noise?
- How is what we have created similar to a duck’s beak?
- Plastic straw
- Push down on the straw to flatten one end and then cut the flattened end into a point.
- Flatten out your straw and then blow into it.
- Feel free to experiment with different amounts of flattening and different point shapes to see how you can adjust the sound.
- When finished, take your duck call into the wild to test it out.
10. Make Ivory Soap Boats

Did you ever carve items out of soap at camp when you were a child? Give your child the same opportunity. Soap can be carved using safe items, like plastic knives.
- Why are we able to carve soap so easily?
- Do you think our boats float?
- Why do you think they float or sink?
- Carving tools (for kids)
- Allow your child to express their creative side by carving their boat out of soap.
- Once they have finished carving it, allow them to test them out in the bathtub. . Extend their learning by discussing density with them–the soap floats because it is less dense than the water.
11. Make Your Own Quicksand
As John Mullaney famously said, “I thought quicksand would be a much bigger problem in my adult life than it would have turned out.” For some reason, quicksand permeates children’s adventure stories – and their imaginations!
- Where can we find quicksand in real life?
- How do you think quicksand works?
- What do you think we will need to make our own quicksand?
- Cornflower (one cup)
- Water (half cup)
- A container
- To make your quicksand, you’ll need to mix the cornflour and water.
- Be sure to stir slowly in order to demonstrate – if you stir too quickly, it will become hard and you won’t be able to see it function the way it should!
12. Make Your Own Lava Lamp

We’ve tried this one in our classrooms, and trust us, our kids go wild year after year. Kids love making something that they can use as home decoration, and they love how easy it is to show new people – this is the experiment that lives on and on!
- How do you think density is involved in this experiment?
- Why don’t the water and oil mix?
- Why can’t we shake our lava lamps?
- Clear Plastic Bottle
- Vegetable Oil
- Food Coloring
- Alka-Seltzer
- Pour water into the plastic bottle until it is approximately one quarter full.
- Then pour vegetable oil in until the bottle is almost completely filled.
- Allow some time for the oil and water to separate.
- It is important that your children do not shake the bottle in this step. It will extend the experiment for no other reason than you waiting for the bubbles to dissipate.
- Add as much food coloring as your child deems fit and then drop a piece of Alka-seltzer tablet into the bottle for the lava lamp fun to begin.
13. Guess the Smell
This one will take a little more prep work, but it’s a great touchstone for your children to begin discussing one of their five senses: the sense of smell!
- What are examples of times we use our sense of smell?
- What other senses do we have?
- If you could only use one sense for the rest of your life, which one?
- Plastic Cups
- Smells (eg. coffee, cinnamon, vanilla, lemon juice)
- Place a variety of common smells in small plastic cups. We like to use coffee, cinnamon, vanilla, and lemon juice.
- Pour these in and place tin foil securely over the top of the cup.
- Poke small holes in the top of the foil.
- Secure the foil with tape (on the sides, not over the holes).
- Allow your children to guess the smells and record their findings on paper.
Home Science Experiments that take 1 to Several Hours
14. mangrove bioshield .
Ecologists and conservationists are pushing for more regulations in building and saving mangrove forests around coastal areas. The reason is represented in this STEM activity. The trees act as a mangrove BioShield (bio = life, shield = protection), showing how natural obstacles can prevent critical damage from marine natural disasters such as tsunamis.
The mangrove BioShield can be for older elementary kids through to high school. Obviously, the younger they are, the more parent involvement. This experiment is done twice to show the effects of having and not having a BioShield. The first part uses little to no trees, and the second uses a forest of trees and rocks.
- What will happen in a tsunami if the village is without a BioShield? And the village with a BioShield?
- Would a BioShield help with hurricanes?
- Would you want to encourage people to save manatee forests if they are beneficial?
- Medium to large clear, plastic container
- Newspaper – wad into balls, then cover half of the bottom container – this help to keep the ground sturdy
- Mud – cover the newspaper and press it in to form a slope down to the empty side of the container. The top side should be flattened for the cardboard houses, then it slopes down into the empty half of the container.
- Cardboard houses (use the bottoms of milk cartons for the house and popsicle sticks for the roofs, place houses on the mud towards the top of the high slope
- Model trees or leafy stems from plants – Different amounts for activity 1 and activity 2
- Several small rocks
- Cardboard – long enough to fit across the container and tall enough to hold it from out of the water
- Water – enough to go halfway up the slope
A tsunami without the mangrove forest – insert only one or two trees down the slope. Place the cardboard piece into the water end of the container and move back and forth to create waves. Notice how easy it is for the water to destroy the village you’ve created.
Repeat the process of constructing the village, but this time insert a lot of trees down the slope to where the water meets the mud. They need to be deeply rooted like mangroves, and I’ve found that aquarium plants work well for this reason. Place the rocks within the mangrove forest and in front of the trees. Add a little bit more water. Insert the cardboard again and move it back and forth to create waves.
15. A Greater Crater
When you look at the night sky and see the moon, one of the first things you notice is its craters. The moon is completely covered in them, and some are so large we can see them from Earth. Meteorites often make the craters that we see when they hit the surface, but it makes us wonder why some craters are so much bigger than others.
This experiment will help you to investigate one of the main reasons why craters come in different sizes.
- What causes craters?
- How big do the meteorites have to be to make a crater?
- What is it about the meteorite that causes the size of the crater?
- Paper to record your results
- Flat floor surface for the experiment, large enough for the newspaper to cover
- Shallow metal pan at least 2 inches deep
- Flour to fill 2 inches of the pan
- ¼ cup hot chocolate powder
- Mesh strainer or flour sifter
- Large marble (and others of varying sizes if comparing results)
- Metric ruler
- Tongs or long tweezers
- Pour the flour into the pan until it reaches 2 inches. Place the pan on top of the newspaper on a level surface.
- Sift a layer of hot chocolate powder over the flour (this is so you can better see the rays and other features of the craters).
- You will be dropping your marble from three different heights, then comparing the sizes of the craters. Measure the diameter (side to side) of the marble and record this on your paper as Marble 1. Hypothesize how large the crater will be and write that next to the diameter of Marble 1.
- Stand next to your pan and hold the marble at knee height above the flour. Drop the marble (do not throw it, just let it fall from your fingers) into the flour and study the shape of the crater. Look for a rim around the crater or any rays coming from the edges.
- Measure across the widest part of your crater, from rim to rim and record on your data sheet as Marble 1 – Knee Height – Width or something similar. You can also draw a picture of your results.
- Very gently use the tongs or long tweezers to remove the marble without destroying the crater.
- Repeat this procedure from waist height, shoulder height, top of head height. Make sure you aim in different parts of the flour so you don’t land on top of another crater. Record all of your results as the different heights you’re using.
- Compare your results.
- You can try again with a different sized marble as “Marble 2” to compare those results with each height as done with Marble 1.
Perhaps now, you’ll look at the moon a little differently!
16. Rube Goldberg Chain Reaction Machine
We’ve all seen them, some pretty far-out there chain reaction machines to complete simple tasks, usually in movies. But they are real , and are becoming even more popular now that we’re all stuck at home for a while. This is a fun way to explore physics with stuff you have at home.
Ask your child to decide what the end goal is (e.g. get the ball into the cup), and ask them to think about creative ways to make it get there. Working together, you can start with small pieces of a circuit to find out how your ball reacts to the set-up, and grow it from there. You can even refer to this video for more ideas:
- What will happen when the ball bounces off of this wall?
- How will these dominoes change the speed of the ball?
- What can we use to make sure that the ball goes in the direction we want it to at this point?
- What should we put here to get the best bounce?
- Paper towel
- Toilet paper tubes
- Fixed objects like walls or furniture
- Any other toys and materials that can be used to build your circuit
To make this a true experiment, it needs to include more than a one-off demonstration, and there are a lot of ways to accomplish this.
- Set up parallel courses and use different sized or weighted balls to go through the circuit.
- Set up one elaborate circuit and use different objects one at a time.
- Set up circuits in different ways to see how different set-ups affect your end goal.
Another experimental component is the process used to create a circuit that reaches your end goal ( like this video about getting the ball into the cup, but you could come up with lots of other endpoints!). Along the way, you and your child get a lot of time to learn about momentum, velocity, friction, energy transfer, and interference (e.g., the cat).
17. Melting

This is a simple and fun experiment that can be set up in a short time and then fill-up your day with observations and new experiments. Using only things you already have at home, you can set-up an engaging experiment with your kids!
Ice melts at different rates depending on a variety of factors including temperature, pressure, and if there are impurities (think salt, sugar, dirt) in the ice or touching the ice. There is a lot of opportunities to get creative and do the experiment in multiple ways, keeping your kids engaged and developing their investigative, experimental, and critical thinking skills.
- Which ice melts the fastest, slowest, and if they have any guesses about why?
- What other ice-melting experiments they think would be fun: Using different temperature liquids? Using different amounts of ice? Different sized cups?
- Lots of ice
- Several matching cups (i.e., they are the same size, shape, and color)
- Measuring cups
- A variety of liquids for the test
- Paper for writing down observations
- Measure the same amount of ice and place it in each cup.
- M easure equal amounts of each liquid and place them in the cups: try to complete this part quickly so that the ice in each cup is in liquid for as close the same amount of time as possible.
- Set up your cups in a place that is easy for your child to watch and observe.
- Ask them to check in at regular intervals (every 15 minutes, every hour) and record or talk to you about their observations.
Other potential experimental examples:
- Using different liquids to test if they affect melting time;
- Using the same liquid and placing ice in different locations to test what conditions throughout your home affect melting;
- Test if different amounts of ice melt at different rates;
- Test if different kinds of cups change melting time.
There are endless possibilities for you to come up with new ways to complete these simple experiments. You get the idea. Explore more!
18. Breathing Leaves
Science experiments don’t get much more simple than this one! It’s effective though and kids will enjoy watching their leaf ‘breathe’. Learning about plant science is often tricky because it can seem a bit abstract. This experiment allows kids to see the process of plants making oxygen right before their eyes!
A question to ask beforehand:
- What do you think will happen if we leave it for a few hours?
- A fresh leaf from a tree
- A bowl of water
- Pluck a fresh leaf from a tree and place it in a bowl of water.
- Use a rock to weigh it down and leave the experiment out in the sun.
- Have your kids predict what they think they will see when they come back in a few hours (they can write their prediction down or draw a diagram if that’s more their style).
- After a few hours, your kids will see lots of tiny little bubbles on the edge of the leaf and in the glass bowl of water (use a magnifying glass to get a closer look if you have one).
So, what’s happening here? Leaves take in carbon dioxide and convert it to oxygen during photosynthesis. The bubbles you can see are the leaf releasing the oxygen it’s created. You could explain to your kids how trees and plants make the oxygen we need to breathe. Kids Fun Science explains this experiment in more detail and suggests taking it further by leaving the plant for a longer period of time (do you see more or fewer bubbles?) or placing a leaf in a dark area to see what difference that makes!
19. How Does Sunscreen Work?

If there’s one thing I know, it’s that kids hate wearing sunscreen! Trying to get it on them is like wrestling a crocodile. Maybe if they knew how sunscreen worked they’d understand how important it is to wear it when they’re out in the sun (and be slightly more cooperative when we’re lathering it over their little faces). This is a simple experiment that shows kids the difference wearing sunscreen will make to their skin.
- What do they observe when they come back?
- Why do they think one side faded and the other not?
- A piece of colored cardboard (a dark color would be best)
- Your usual bottle of sunscreen
- Have your kids smear the sunscreen over one part of the cardboard and leave the other part clear.
- Kids can then predict what they think will happen when they return to the experiment after a few hours.
- Talk to them about how the sun’s UV radiation is absorbed by the sunscreen so it can’t get through to damage the cardboard.
You could even take it further by trying different kinds of sunscreen or leaving your cardboard out during different times of the day.
20. Make A Rubber Egg
Imagine a world in which eggs can be used like bouncy balls. Well, with a couple of home supplies and a little bit of science, you can live in that world. Your child will be dazzled as they remove eggshells from eggs while leaving the insides intact.
- Is vinegar an acid or a base?
- Is there another substance that could do this?
- Simply leave the egg in the vinegar for a few hours and wait to see what happens. Because of the transformative nature of this experiment, it lends itself to science journaling.
- Consider having your kiddos draw before and after pictures of the eggs in order to track their journeys.
21. Flying Tea Bags

Nothing will get your kids’ attention faster than telling them that you are going to spend some time creating something that will fly. However, because this experiment will involve fire, please ensure that you select a time in which you will be able to provide ample adult supervision.
- How do we stay safe with fire?
- How do we make sure we don’t damage the surface we are working on?
- Why do you think the tea bag will fly?
- Single Serving Tea Bags
- A Small Bowl
- A Non-Flammable Work Surface
- First, open the tea bags and unfold them.
- Empty the leaves from the bag.
- Stand the tea bags up on your surface and light the top of each bag on fire.
As they begin to burn, they will float into the sky!
22. Make Wax Paper Lanterns
Your children will love the chance to display their fantastic art skills by creating these paper lanterns. If you want to add a culture lesson, have your children research German’s St. Martin’s Day and learn about why children parade through the streets with lanterns. We promise there’s a good moral story involved here!
- When could we use lanterns?
- What safety considerations do we need to use in this project?
- Why can we see the light through the wax paper?
- Popsicle Sticks
- To begin, tear a ten-inch piece of wax paper off of the roll and cut it in half.
- After that, fold each piece in half.
- Allow your child to color their image on top of the wax paper. (This is a great place for an impromptu lesson in color mixing).
- Fold the wax paper and iron it (consider something in between the crayon mess and the iron you use on your clothes).
- Finally, glue the craft sticks into squares, add the wax paper, and turn it into a cube.
Voila, you’ve created your own lantern!
23. Create an Insect Habitat
Alright, this one isn’t for the faint of heart. Draw up your courage and send your child into the backyard to collect all of the creepy crawlies they’d like to.

Now you have a home for them. Better yet, you can keep your child entertained for hours as they track the growth of their bug friends.
- What do bugs need to survive?
- What do bugs eat?
- What is the difference between a need and a want?
- Imagination
- Find something that you’re willing to sacrifice to the bugs in order to create a habitat for them – we recommend a shadowbox so that your child can see inside, but a cardboard box will do just fine as well.
- Ensure that there is breathing room for the bugs.
- Create a habitat with sticks, bark, small rocks, dried leaves, and whatever else you can find.
- If you’re willing to hang onto the habitat long enough, use it as an opportunity to talk about decomposition as the bugs begin to break down the twigs.
Long-Term Science Experiments at Home
24. crystal kingdom.
This is the oldest trick in the book, but it’s popular because it’s so effective, fun, and has great results. The only drawback to most crystal-growing recipes is that they take ages to grow, and to be quite honest this one is no exception. In fact, these crystals will take several days to grow but the end result is worth it. The reason is that this experiment involves growing a whole landscape of beautifully colored salt and bluing crystals. Here’s a video for visual reference:
A few things to keep in mind: Allow for plenty of air circulation, preferably inside rather than outside. Ammonia is not necessary but does help in the process.
- What will happen when you add ammonia?
- Why does more salt and less liquid create faster crystallization?
- What part does the bluing solution have in crystal growing?
(Answers can be found here )
- Two bottles of bluing solution
- Large tray/cookie sheets with sides
- Measuring cup
- Liquid watercolors
- Eye droppers
- Cut sponges into large pieces. Spread them out on the tray.
- Measure out 1 cup of each of salt, water, and bluing and then gently mix together.
- Evenly coat or sprinkle the mix over the sponges.
- Add 1 cup of ammonia to the sponges.
- Coat an extra 1 cup of salt on to the sponges.
- By now you’ll see some crystals growing . Sprinkle the magic mix again: 1 cup each of salt, water, and bluing. You can pour the ingredients onto the tray instead of on top of the crystals to keep them from breaking. Don’t worry, more will grow!
- Take an eyedropper, and drop a tablespoon of each liquid watercolor (undiluted) in different patterns over the sponges and crystals.
- Take note of your garden and what the crystal formations look like. You can make a sketch in your notebook as a before and after. Ask questions and observe!
- Observe how the crystals are bigger than before, and notice the colors aren’t as vibrant. Compare the differences in shapes, sizes, and colors.
- If you want more crystals to grow, add a little more water, bluing, and salt.
25. Blow up a Balloon with Yeast
We are surrounded by science in action, but sometimes it is really difficult to see what is happening, especially when it is on a small-scale. When we make bread, yeast ‘eats’ the sugars in the food and creates CO2, giving bread its airy texture. This experiment lets you both visualize what happens when yeast consumes sugar and is a great set-up for an experiment that can be observed throughout the day.
Depending on your supplies and time, you could start with a demonstration and use that to think of other tests, or you could set up several parallel tests at the same time.
- How quickly does the balloon filled with air?
- When does it stop filling (at some point the yeast will run out of food and will stop making gas)?
- Does the starting temperature affect the experiment?
- Does the balloon fill faster in different places in your home (try especially for different air-temperatures, you could include an outside location)?
- Some balloons
- Blow up the balloon a few times before starting so that it’s loosened up a bit.
- Fill the bottle with about 1 inch of warm water (heat is required to activate the yeast, but you could experiment with different temperatures), add the yeast and swirl to dissolve.
- Add the sugar and swirl more.
- Place the balloon over the opening to the bottle and wait. You should expect to see the balloon begin to inflate after around 20 minutes.
- Continue checking and observing how much the balloon inflates throughout the day.
More example experimental setups include:
- Do different temperatures – either with the water you start with or the air the yeast lives in – affect how quickly the balloon blows-up?
- Does using 2x the yeast result in a balloon that is 2x bigger, or blows-up 2x faster?
- Do different types of sugar (e.g., white sugar, honey, syrup, flour) affect how quickly the balloon blows up or how big it gets?
A sk your child to think of new experiments (you could prompt with some of the examples above, or ideas from this post ).
26. Seed Germination
A really simple but fun multi-day experiment is germinating seeds under different conditions. This means finding some quick-sprouting seeds such as beans and putting them in different conditions to see how that affects germination (sprouting leaves and roots) and growth.

I love using seed experiments because they are inexpensive, simple, and leave a ton of room for creating your own unique experiment.
- Which seed will sprout fastest?
- Seeds (Beans, radishes, squashes, and many flowers sprout quickly from large seeds, making them good choices.)
- Small pots or paper cups
- Potting soil
- Cloth or paper towel
- Somewhere with good light
- To get started, you’ll need some seeds – feel free to choose something you already have, if you’re a gardener you might have some seeds ready for the coming season and could spare a few – or find something online or at your local nursery.
- Use small pots or paper cups and fill each with your growth material (we recommend a minimum of 3 for a useful comparison).
- Fill one with potting soil, one with sand, and one with a cloth or paper towel.
- Place them somewhere with good light, and add water.
- Ask your child to predict which seed will sprout fastest, and make observations every day. If possible, make them around the same time each day.
- Once you see growth, you can ask your child what they think caused any differences, and you can use that as a jumping-off point for more experiments
Additionally, you could:
- Use one type of seed and different types of growth media: soil, paper towel, gravel, sand, water, etc.
- You could use different seeds (beans, flowers, grass, herbs) and grow them under the same conditions (soil, water, sun exposure) to see how different plants grow differently.
- You could see how different light conditions (by a window, in the basement, in a bright room away from a window, etc.) affect germination.
You could also extend each experiment by simply continuing to grow each seed to learn whether the different germination time affects long-term growth (you may want to re-pot everything in the soil for this to be effective, depending on the specifics of your initial experiment).
27. Colored Celery

It’s hard to imagine plants having little capillaries inside them that transport water and nutrients, but this experiment shows that in action. It’s easy to set up, but you’ll have to wait at least a day to see some results. Your kids will be able to see how transpiration takes place and plants absorb water from the soil all the way up into their leaves.
- A few stalks of celery (celery works best for this because it’s a bit more visible, but you could also use flower stems)
- Different food coloring
- Place each stalk in a cup of colored water and make your predictions about what will happen.
- After a day or so you’ll see the celery leaves becoming the color of the water they’re standing in.
- Have your kids describe their observations (they can write down what they see or draw it if they prefer).
- If you look at the base of the stem you’ll also see tiny little holes that the colored water is traveling through.
When you’re done with the experiment, make sure you snap the celery and look inside – you should be able to see the capillaries in action. For more ideas, Little Bins for Little Hands has got some great hints and tips for this experiment.
28. Moldy Bread
This experiment is an oldie, but a goodie! Kids love looking at disgusting things and this one will certainly come up with the goods. Not only will kids learn about how mold grows, but they might also take on some lessons about the importance of washing their hands!
You might want to check out the results of this experiment at Science Alert before you start to see if your stomach is up to it.
- A few slices of bread
- Some ziplock bags
- Sticky little hands.
- Get a few slices of bread and lay them out on your kitchen bench.
- Have your kids touch one piece of bread with dirty, unwashed hands.
- They can wash their hands with soap and water and touch another slice, then do the same using hand sanitizer.
- Leave one piece of bread untouched.
- Place them all in clear, labeled ziplock bags and predict which one will grow the most mold.
- Leave your bread slices for at least a week (it may take a bit longer, depending on the conditions where you live) and get the kids to record their observations.
You can also try wiping your bread slices on other surfaces to see what moldy results you get (their laptop or tablet is a great place to start)!
29. Sprouting Beans

Give your household a real survivalist feel by beginning an indoor garden. We recommend planting your beans in a clear cup so that your children can be privy to all of the processes during the plant’s journey.
- How does a plant grow?
- What does germination mean?
- What is in season to grow in our area now?
- Unprocessed Beans
- If you’d like your child to see every step of the process, consider placing the beans inside of a damp paper towel inside of a ziplock.
- You can wait, see the germinated seed together, and then plant it inside of a small cup.
- Once inside the cup, watch it grow.
Extend your work by planting various beans and altering the growth conditions in order see what makes your beans grow best!
30. Begin Composting
Begin your “go green” resolutions by teaching your child the value of composting! Best of all, once the science experiment is done, your family will have a recycling process that will last your entire lifetimes.
- Why is composting important?
- How else can our household go green?
- Why do we need a foundation layer for compost?
- Compost Bin
- Organic Material
- First, create a compost bin. You can purchase one or build one out of wood.
- To begin your composting, you’ll need even amounts of brown materials (think shredded paper, dryer lint, etc.) and green materials (think fruit and vegetable waste, lawn clippings, etc.).
- If you’re really feeling fancy, throw some earthworms in there.
For days to come, your family will be able to discuss what can and cannot be broken down by the decomposers inside of the compost bin. Never-ending science!
31. Turn Grapes Into Raisins

Your kids may or may not eat raising – but we can guarantee you, they’ve likely never considered the option of creating their own!
- What other snacks can we make with science?
- Should we ever eat our experiments?
- How does this work?
- For this experiment, you’ll need grapes. (Really, that’s it!)
Leave your grapes somewhere where they will not be disturbed and use this as an opportunity for your children to journal the changes in the grapes from day to day. Believe it or not, this type of sequential journaling is a valuable literacy skill!
32. DIY Science Experiment
The best science experiment your child can engage in is the one they create themselves! Begin brainstorming a list of questions and let the world be their oyster as they plan and carry out their own experiments. Some of our favorite brainstorming questions, from Scholastic’s Science-Fair Project Guide, are listed below:
- What is the effect of toothpaste brand on teeth-cleaning power?
- What brand of trash bag can withstand the most weight before ripping?
- How does the type of material affect how long a shirt takes to dry?
Written by Miranda Altice, Kaitlin Anselmo, Mark Coster, Allison Ebbets, and Jodie Magrath.

Mark is the driving force behind STEM Geek. With 20 years of experience in chemistry education and research, and 3 willing children as guinea pigs, Mark has a passion for inspiring kids and adults to combine fun and learning with STEM Toys!
Editor’s Picks

7 Best LEGO Star Wars Sets | Our Top Picks of All Time!

Best LEGO Creator Sets – Take Your Pick From These 7 Gems!

How to Use a Metal Detector: 8 Essential Tips to Get the Most of It

Best Metal Detector for Kids: 5 Top Picks (+ Buying Guide)

Best 2+ Player Cooperative Board Games (Top 6 in 2024)

MEL Chemistry Review: Is Your Child the Next Bill Nye?
45 Easy Science Experiments for Kids
Hello, STEM! These simple DIY activities can be done at home or in school.

We've been independently researching and testing products for over 120 years. If you buy through our links, we may earn a commission. Learn more about our review process.
Imagine blowing the biggest bubbles imaginable — or even making bubbles within bubbles. Or sending vessels — rockets, tea bags, airplanes — soaring through the sky for impossible distances. Now imagine making things explode, or change colors, or reveal hidden messages with just a few simple mixtures.
None of this is magic. It's all science that you can do at home, most likely with ingredients you already have in your house. So, next time you need a boredom-busting indoor activity on a rainy day or a DIY project to get their minds humming, try one of these best at-home science experiments for kids , which cover topics like cover magnetism, surface tension, astronomy, chemistry, physics and more.
First off, it's good to start them off with the scientific method. Give them a journal to record their observations, questions, hypotheses, experiments, results and conclusions. As always, safety counts: wear goggles and coats or aprons if need be (sometimes kids get a kick out of how scientific the protective gear makes them look), and always make sure that the kids are supervised when doing them. (Warning: Some of these are messy!)
These experiments are mostly designed for preschoolers through elementary schoolers — with a couple that are either demonstrations or better for older kids — but if you have a younger one, you can check out these 1-year-old learning activities , toddler learning activities and preschool/kindergarten learning activities , some of which also cover STEM subjects.
Floating Fish

Here's another one that deals with solubility and density.
- Draw the outline of a fish on the bottom of a glass plate or tray in dry-erase marker. Retrace your drawing to make sure all the lines are connected. Let dry for a minute or two.
- Fill the measuring cup with tap water. Place the pour spout just inside the corner of the dish and add water very slowly until it just covers the bottom. Be careful not to pour water directly onto your drawing or make splashes near it. The water will move toward your drawing, eventually surrounding it. Observe what happens. If the water splashes or it doesn’t work on your first try, empty the dish, erase the drawing with a paper towel, dry off the dish, and try again.
- Tilt the dish slightly from side to side. What happens? Jot it down.
The ink in dry erase markers is engineered to be slippery. It’s made with a chemical that causes it to easily release from surfaces. (Permanent markers are made with a chemical that makes the ink stick to surfaces, so be sure not to use these in your experiment!)
The easy-release ink lets go from a surface, but why does it float? There are two reasons. First, dry erase ink isn’t soluble, which means it won’t dissolve in water. Second, dry erase ink is less dense than the water, so it becomes buoyant, meaning it can float. When you tilt the dish, the fish moves around on the water’s surface.
From Good Housekeeping Amazing Science: 83 Hands-on S.T.E.A.M Experiments for Curious Kids! See more in the book »
Brush, Brush!

This one will really get them into brushing their teeth once they scientifically prove all the good things that toothpaste can do.
- Write on sticky notes: Soda 1, Soda 2, Juice 1, and Juice 2. Place them in a row on a counter.
- Fill two glasses halfway with brown soda and place behind the Soda 1 and Soda 2 sticky notes. Fill two glasses halfway with lemon juice and place behind the Juice 1 and Juice 2 sticky notes.
- Carefully place one egg in the bowl. Squeeze a big dollop — about one tablespoon — of toothpaste on top of the egg and gently rub the toothpaste all around with your hands until the egg is completely covered in a thick layer of toothpaste. Repeat with a second egg.
- Gently submerge the toothpaste-covered eggs into the liquids: one egg in the glass labeled Soda 1 and the other egg in the glass labeled Juice 1. Wash and dry your hands.
- Gently submerge the remaining eggs, without toothpaste on them, in the remaining glasses: one in the glass labeled Soda 2 and the other in the glass of juice labeled Juice 2. Wash and dry your hands. Leave the eggs in the glasses for 12 hours.
- After 12 hours, remove the eggs from the glasses of soda one at a time. Rinse them in cool water and pat them dry with the towel. Place each egg by the sticky note of the glass it was in. Are the eggs the same or different colors?
- Remove the eggs from the glasses of juice one at a time. Rinse them under the faucet and pat them dry. Place each egg by the sticky note of the glass it was in. Feel the eggs gently. Does one feel stronger or weaker than the other?
- Write down your observations in your science notebook.
The eggshells in this experiment represent the enamel (outer coating) on your teeth. Toothpaste cleans your teeth and prevents stains: it removes food and drink particles that are stuck on your teeth. Teeth can be stained easily by dark-colored liquids like cola, coffee or tea. The egg without toothpaste will be brown and discolored. The egg covered in toothpaste was protected from turning brown.
Toothpaste also protects your pearly whites from decay (breaking down). The egg without toothpaste left in the lemon juice was worn down and soft to the touch, while the egg that was protected with toothpaste is stronger. The lemon juice is acidic, and those acids broke down the shell just as acidic drinks can wear away your tooth enamel. When a tooth is worn down, a cavity can form more easily. But the fluoride in toothpaste mixes with your saliva to create a protective coating around your tooth enamel. It helps keep your teeth strong and cavity-free.
Grow an Avocado Tree

For an easy lesson in Earth Science, your family can grow an avocado tree from a pit. You can buy an AvoSeedo kit , or just peel the seed and suspend it over water with toothpicks.
Get the tutorial »
Milk Bottle Xylophone

No for an experiment in sound!
- Arrange six glass jars or bottles, all the same size with no lids, in a line. What will each jar sound like when you tap it with a spoon? Make a prediction, then tap each jar. Record your observations.
- Next, put water in each of the jars. Pour 1⁄4 cup (60 ml) of water into the first jar. Add 1⁄2 cup (120 ml) of water to the second jar. Continue in 1⁄4-cup increments, adding 3⁄4 cup (180 ml) of water to the third jar, 1 cup (240 ml) of water to the fourth jar, 11⁄4 cups (300 ml) of water to the fifth jar, and 11⁄2 cups (360 ml) to the sixth jar. Add a couple of drops of food coloring to each jar.
- What will each jar sound like? Will they sound the same or different than when the container was empty? Will they sound the same or different from one another? Record your predictions.
- Tap each jar with a metal spoon. Write down your observations about each jar’s pitch (how high or low a sound is) in your notebook.
Sound waves are created by vibrations, which are back-and-forth movements that are repeated again and again. Pitch depends on the frequency of the waves — how many are created each second. A high pitch is created by high-frequency sound waves, and can sound squeaky. A low pitch is created by low-frequency sound waves, and sounds deep and booming.
When you tapped the jar, it vibrated. The vibrations traveled from the jar to the water to the air and eventually to your ears. The jars with more water had a low pitch. The sound waves vibrated more slowly because they had more water to travel through. The jars with less water had higher pitches. The sound waves vibrated faster because they had less water to travel through. A jar with no water in it makes the highest pitch because it has the least substance to travel through.
"Elephant Toothpaste"

Okay, elephants don't really brush with this stuff, which is made from a chemical reaction between hydrogen peroxide, yeast, dish soap and a few other simple ingredients. But this experiment has a big "wow" factor since, when the substances are mixed, the "toothpaste" foams out of the bottle. You can use it to teach kids about catalysts and exothermic reactions.
Get the tutorial at Babble Dabble Do »
DIY Compass

Explore the way magnetism works, and how it affects everyday objects, by magnetizing a needle and making a DIY compass. You can even spin the compass in the water, and it'll end up pointing the right way again.
Get the tutorial at STEAM Powered Family »
Craft Stick Chain Reaction

Kids can learn about the differences between potential and kinetic energy with this chain reaction. It makes a big impact: Once the tension is released, the pom poms go flying through the air!
Get the the tutorial at Science Sparks »
Color-Changing Invisible Ink

Kids will feel like super-spies when they use this heatless method to reveal pictures or colors written with "invisible ink." You can try different acid/base combinations to see which one makes the most dramatic result.
Get the tutorial at Research Parent »
Paper Bridge

Get the engineering back into STEM with this activity, which challenges kids to create a paper bridge that's strong enough to hold as many pennies as possible. How can they manipulate the paper to make it sturdier? (Hint: Fold it!)
See the paper bridge tutorial at KidsActivities.com »

Challenge your little scientist to lift up an ice cube with just a piece of string. It's possible ... with a little salt to help. Salt melts the ice and lowers the freezing point of the ice cube, which absorbs the heat from the water around it, making the water cold enough to re-freeze around the string.
Get the tutorial at Playdough to Plato »
Marshmallow Catapult

Another lesson in potential and kinetic energy, kids will love sending mini marshmallows flying in the name of science. Change some of the variables and see how that affects the marshmallow's trajectory.
Get the tutorial at Hello, Wonderful »
Leaf Breathing

It's hard for kids to picture how plants and trees "breathe" through their leaves — until they see the bubbles appear on a leaf that's submerged in water. You can also teach them about photosynthesis by putting different leaves in different spots with varying levels of sunlight.
Get the tutorial at KC EDventures »
Hoop-and-Straw Airplane
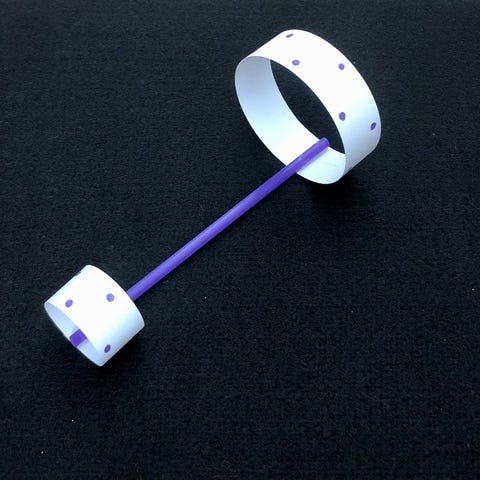
We all remember how to fold those classic, triangular paper airplanes, but these hoop-and-straw airplanes fly way better (and straighter). Experiment by changing the length of the straw and the size of the hoops and see how it affects the flight.
Get the tutorial at Mombrite »
Film Canister Rocket

Blast off! You don't need jet fuel to make these rockets go, just Alka-Seltzer tablets and baking soda, but they'll be amazed when they achieve lift-off! (Note: If you can't find old film canisters, tubes of Airborne work, too.)
Get the tutorial at Raising Lifelong Learners »
Coin Inertia

Stack up about five or so coins on a piece of cardboard and place it over a glass of water. Then, flick the cardboard out from on top of the glass. Do the coins drop into the water, or ride with the cardboard? Due to inertia, they drop into the water — a very visual (and fun!) demonstration of Newton's First Law of Motion.
Get the tutorial at Engineering Emily »
Apple Oxidation

What works best for keeping an apple from turning brown? Test to find out! Slice up an apple, and let each slice soak in a different liquid. Then take them out, lay them on a tray, and check the brownness after three minutes, six minutes and so on. Not only does this test the properties of different liquids, it also helps students practice the scientific method if they create hypotheses about which liquids would be most effective.
Get the tutorial at Jennifer Findley »
RELATED: 50 Fun Activities for Kids Will Keep Them Entertained for Hours
Coffee Ground Fossils

By making a salt dough with coffee grounds and pressing various shapes into it (toy dinosaur feet, seashells), kids can get a better understanding of how fossils are made. If you poke a hole in the top before it dries, the kids can hang their "fossils" up in their rooms.
Get the tutorial at Crafts by Amanda »
Chromatography Flowers

Chromatography is the process of separating a solution into different parts — like the pigments in the ink used in markers. If you draw stripes around a coffee filter, then fold it up and dip the tip in water, the water will travel up the filter and separate the marker ink into its different pigments (in cool patterns that you can display as a craft project). This family made the end-result even brighter by adding an LED circuit to the center.
Get the tutorial at Steam Powered Family »
Water Walking

You'll need six containers of water for this one: three with clear water, one with red food coloring, one with blue coloring, and one with yellow coloring. Arrange them in a circle, alternating colored and clear containers, and make bridges between the containers with folded paper towels. Your kids will be amazed to see the colored water "walk" over the bridges and into the clear containers, mixing colors, and giving them a first-hand look at the magic of capillarity.
Get the tutorial at Fun Learning for Kids »
Sunscreen Test

This experiment puts the A (art) in STEAM: Paint different designs on construction paper with different sunscreens, leave the papers out in the sun and compare the results. Then, hang your "conclusions" on your fridge.
Get the tutorial at Tonya Staab »
Marisa (she/her) has covered all things parenting, from the postpartum period through the empty nest, for Good Housekeeping since 2018; she previously wrote about parents and families at Parents and Working Mother . She lives with her husband and daughter in Brooklyn, where she can be found dominating the audio round at her local bar trivia night or tweeting about movies.

Parenting Tips & Advice

Ground Rules for Talking About Politics With Kids

How to Raise a Good Citizen (A Guide for Parents)

The 50 Best Books to Stock Your New Baby's Library

10 Things You’re Doing Right as a Parent of a Teen

40 Fun Indoor Activities for Kids

250 Short Baby Boy Names That Are Perfectly Unique

150 Popular Middle Names for Baby Boys

100 Cool Grandpa Names Your Grandkids Can Use

Research-Backed Ways to Raise a Reader

How to Throw a Birthday Party for a Picky Teen

150 Fun Questions to Ask Kids at Dinnertime

7 Things NOT to Do As a New Grandparent
BIOCHEMINSIDER
130 Laboratory Apparatus And Their Uses (With Pictures)
A laboratory is a special room or place that is equipped to facilitate scientific experiments, observations and for teaching science. Laboratory apparatus refers to the various tools, equipment, and instruments used in scientific research, experimentation, and analysis within a laboratory setting. These tools are essential for conducting experiments, measuring and analyzing data, and ensuring the accuracy and reliability of scientific results.
Some of the laboratory apparatus are used as a source of heat, for safety, for making observations and for measurement of variables such as voltage, temperature, volume, time and mass.
There are apparatus that are used in general laboratory experiments while others serve specific in experiments. They are also made from materials that are resistant to chemical reactions and corrosion. Common materials include glass, stainless steel, and various types of plastics.
It is important to note that most of the apparatus that are used as containers or reaction vessels are made of transparent glass or plastic and may come in different sizes. Let us talk about Laboratory apparatus in three categories: Basic Apparatus, Safety Apparatus , General Apparatus and Specialized Apparatus
Here is a list of 130 laboratory apparatus / Equipment
General equipment/apparatus that are found in almost all laboratories:
- Alcohol burner
- Bunsen burner
- Burette clamp
- Buchner funnel
- Balance scale
- Conical or titration flask
- Crucible tong
- china dish (Evaporating Dish)
- Crucible with cover
- Clay Triangles
- Dry-cell battery
- Dissecting set
- Erlenmeyer flask
- Flat bottomed flask
- Filter paper
- Friability tester
- Glass funnel
- Glass tubing
- Litmus paper
- Measuring cylinders
Mortar and pestle
- Measuring flasks
- No of weights
- Petri dishes
- Rubber stopper
- Reagent bottle
- Rubber tubing
- Stirring rod
- Separatory funnel
- Stethoscope
- Speedometer
- Test tube rack
- Tripod stand
- Test tube holder
- Test tube stand
- Test tube brush
- Tuning fork
Thermometer
- Wash bottle
- Watch glass
Others Laboratory Apparatus or Equipment
- Analytical balance
- Atomic absorption spectrometer
- BOD incubator
- Chromatography column
- Cryogenic freezer
- Colorimeter
- Conductivity meter
- Dewar flask
- Distillation apparatus
- Electrophoresis chamber
- Flame photometer
- Gas chromatograph
- Geiger-Muller counter
- Inoculating loop
- Inverted microscope
- Kjeldahl apparatus
- Laboratory oven
- Laboratory refrigerator
- Laser spectrometer
- Magnetic stirrer
- Mass spectrometer
Microcentrifuge
- NMR spectrometer
- Orbital shaker
- Oscilloscope
- Particle counter
- PCR machine (Polymerase Chain Reaction)
- Peristaltic pump
- pH electrode
- Pipette filler
- Polarimeter
- Refractometer
- Rotary evaporator
Spectrophotometer
- Syringe filter
Ultracentrifuge
- UV-Vis spectrophotometer
- Vortex mixer
- X-ray diffraction machine
- YSI meter (for measuring dissolved oxygen)
- Gas syringe
- Melting point apparatus
- Infrared spectrometer
- Particle size analyzer
- Bacterial incubator
- Thermal cycler (PCR machine)
- Gas manifold
- Conductivity cell
- Reflux condenser
- Freeze dryer
- Inert gas chamber
- Ultrasonic cleaner
- Atomic force microscope (AFM)
- Gas generator
- Digital pH meter
- Atomic emission spectrometer
- Magnetic balance
- Tensiometer
- Ultraviolet lamp
- Inoculation needle
- Rotary shaker
- Autotitrator
- Freeze-thaw chamber
- Gel documentation system
- Pipette tips
- Rotary vane pump
- Vacuum desiccator
- Gas chromatography-mass spectrometry (GC-MS)
- High-performance liquid chromatography (HPLC) system
- Inverted fluorescence microscope
Basic Laboratory Apparatus
Bunsen Burner
This is a piece of apparatus that is used as a safe source of heat in laboratories using a single gas flame. A Bunsen has an inlet that is usually connected to an external source of laboratory gas by rubber tubing. Its flame is used not only for heating, but for combustion and sterilizing objects too.
This is an apparatus that is used to give finer details of small objects that would otherwise not be seen by the naked eye or a hand lens. It does so by magnifying objects up to thousands times their original size. There exist two main variants of a microscope namely; a light microscope and an electron microscope
Weighing Balances
These are used to weigh the mass of substances in a laboratory. There are different types of weigh balances such as beam balance, spring balance, top pan balances and electronic balances.
Watches and clocks
These are apparatus for measuring time. Stop watches and stop clocks are the most commonly used for accurately measuring time during experiments.
When it comes to measuring the voltage between any two points, nothing does the job better than a voltmeter. It is normally connected in parallel with a device so as to measure its voltage.
Beakers serve a wide range of purposes. Calibrated beakers are used to measure approximate volumes of liquids, holding both liquids and solids and heating them when necessary. In addition to that, beakers may be used for stirring and mixing different substances in a laboratory.
Volumetric Flask
Volumetric flasks come in handy when fairly accurate and precise volumes of liquids are required. They can as well be used for dilution when preparing standard solutions.
This is an apparatus that is used for adding fairly accurate volumes of liquids up to nearly 0.01ml especially during titrations. It is fitted with an adjustable stopcock that regulates the amount of liquid that is released at a time.
A pipette (sometimes spelled pipet ) is a laboratory tool commonly used in chemistry, biology and medicine to transport a measured volume of liquid, often as a media dispenser. Pipettes come in several designs for various purposes with differing levels of accuracy and precision, from single piece glass pipettes to more complex adjustable or electronic pipettes.
A thermometer is used to measure the degree of hotness or coldness of a substance. They come in different types such as maximum and minimum thermometer, clinical thermometer and general purpose thermometers.
Flat-bottomed Flask
It is used for general laboratory experiments. A flat-bottomed flask can be used to collect, measure and hold liquids. They may as well be used for heating substances and mixing solutions in a laboratory.
Filter Funnel
Filter funnels are used for delivering different amounts of liquids carefully into holding apparatus. It can also be used together with a filter paper to separate finer solid substances from liquids. They vary in sizes and material from which they are built from depending on the purpose for which they are needed.
A desiccator is a sealable storage unit used for drying or keeping moisture sensitive substances free from moisture. There are two main types of desiccators that are made from polycarbonate or polypropylene material. These are; vacuum desiccators and non-vacuum desiccators.
Reagent Bottle
Reagent bottle or media bottle refers to containers used for storing and sampling both liquid and solid bench reagents in a variety of laboratory experiments. Most reagent bottles are made of glass or plastic.
A spatula is a broad, flat, hand-held blade apparatus that is used for spreading, mixing and scooping solid substances. The do come in various shapes and sizes.
Dropping funnel
This is an apparatus that is used to add controlled amounts of liquids into reaction vessels more so when the reaction is expected to be too vigorous if large amounts of the reagent are used at a go.
These apparatus are used to prepare solid reagents into a paste or powder by grinding, crushing or pounding them. They are mostly made of metal, wood, nonporous marble and granite material.
Test-tube is a tubular apparatus that is used for general laboratory experiments. They may be used to hold and compare chemical substances. In addition to that, test-tubes can be used to mix liquid substances and heating small chemical samples.
This is a heat resistant apparatus used when heating solid substances under high temperatures. It is commonly made of porcelain as it is resistant to heat when strongly heating solid substances.
Safety Apparatus
It is essential for any laboratory to have a wide range of safety equipment at its disposal. They are intended to keep laboratory users and their working environment safe from injuries, corrosive chemicals, poisonous fumes or accidental fires while carrying out experiments. The list of protective gear ranges from:
Safety Goggles
- Purpose : Safety goggles provide eye protection by shielding the eyes from chemical splashes, flying debris, and hazardous fumes or liquids.
- Usage : They are essential in laboratories, workshops, and industrial environments where eye hazards are present. Goggles should fit snugly to prevent entry of harmful substances.
Disposable Coveralls and Aprons
- Purpose : Disposable coveralls and aprons are protective garments that shield the body and clothing from chemical spills, contaminants, or biohazards.
- Usage : Workers wear these items to prevent exposure to hazardous substances, ensuring both personal safety and contamination control.
Disposable Latex Gloves
- Purpose : Disposable latex gloves are worn to protect the hands from contact with chemicals, biological materials, and contaminants.
- Usage : These gloves are common in laboratories, healthcare settings, and industries where hand protection is essential. They reduce the risk of skin contact and contamination.
Plastic Bags
- Purpose : Plastic bags are used for containing and disposing of hazardous waste materials, contaminated items, or biohazards.
- Usage : In laboratories and medical facilities, plastic bags are crucial for safe disposal of waste materials and maintaining cleanliness.
- Purpose : Gas masks protect the respiratory system by filtering out harmful gases, fumes, and particulates from the air.
- Usage : Gas masks are used in environments where there is a risk of exposure to toxic or hazardous airborne substances, such as during chemical spills or in industrial settings.
Fire Blanket or Extinguisher
- Purpose : Fire blankets and extinguishers are used to suppress fires in emergency situations.
- Usage : In the event of a small fire, fire blankets can be used to smother flames. Fire extinguishers are designed to spray fire-suppressing agents to extinguish fires safely.
First Aid Kits
- Purpose : First aid kits contain essential medical supplies and equipment to provide immediate medical assistance in case of injuries or accidents.
- Usage : First aid kits are located in workplaces, laboratories, and public areas to address injuries, burns, cuts, and other medical emergencies.
Plumbed Eyewash Units
- Purpose : Plumbed eyewash units provide a continuous flow of water to rinse and flush the eyes in case of chemical exposure.
- Usage : Eyewash stations are installed in laboratories and workplaces where hazardous chemicals are handled, ensuring prompt eye irrigation in case of accidents.
Flammable Safe
- Purpose : A flammable safe is designed to store flammable liquids and materials safely, preventing ignition or explosions.
- Usage : These safes are essential for fire safety in laboratories, where flammable substances are often used or stored.
Chemical Spill Kits
- Purpose : Chemical spill kits contain materials and equipment for responding to chemical spills, containing and neutralizing the spill, and protecting personnel.
- Usage : In laboratory environments, chemical spill kits are crucial to mitigate the effects of accidental chemical spills, preventing harm and environmental damage.
Plastic Dust Pan and Scoop
- Purpose : Plastic dust pans and scoops are used to collect and safely dispose of solid chemical spills, dust, or debris.
- Usage : They are essential tools for cleaning up laboratory or industrial workspaces, ensuring the safe removal of potentially hazardous materials.
General Laboratory Apparatus
- Purpose : Microscopes are used to magnify and visualize objects or specimens that are too small to be seen with the naked eye. They are essential tools in fields such as biology, microbiology, and materials science.
- Components : A typical microscope consists of an eyepiece, objective lenses with varying magnification powers, a stage for holding the sample, and a light source for illumination.
- Usage : Researchers place a sample on the stage, adjust the focus using the fine and coarse adjustment knobs, and select the appropriate objective lens for the desired magnification.
- Purpose : Bunsen burners are used for heating, sterilizing, and flame-related experiments in the laboratory. They provide a consistent open flame.
- Components : A Bunsen burner has a gas inlet, an adjustable air vent, and a flame nozzle.
- Usage : The flame intensity and type (oxidizing or reducing) can be adjusted by controlling the air mixture. Bunsen burners are commonly used in chemistry for tasks like heating solutions and sterilizing equipment.
- Purpose : Beakers are used for holding, mixing, and heating liquids. They come in various sizes and are a staple in laboratories for general-purpose tasks.
- Features : Beakers typically have volume markings, a spout for pouring, and a flat bottom.
- Usage : Beakers are versatile containers, but they are not designed for precise measurements. They are often used for mixing solutions, conducting simple reactions, or as a vessel for holding liquids during experiments.
Erlenmeyer Flask
- Purpose : Erlenmeyer flasks are conical-shaped containers with narrow necks. They are used for mixing, heating, and storing liquids, particularly when you need to prevent splashes and evaporation.
- Features : Erlenmeyer flasks have volume markings and can be fitted with stoppers or caps.
- Usage : They are commonly used for titration, as reaction vessels for chemical reactions, or as containers for cultures in microbiology.
- Purpose : Test tubes are small, cylindrical containers used for holding, heating, or mixing small quantities of liquids or solids.
- Features : They come in various sizes, and some have screw caps or stoppers.
- Usage : Test tubes are versatile and widely used in chemical and biological experiments, such as holding reagents, conducting small-scale reactions, or culturing microorganisms.
Graduated Cylinder
- Purpose : Graduated cylinders are used to accurately measure the volume of liquids. They have volume markings for precise measurements.
- Features : They have a narrow, graduated scale and a spout for pouring.
- Usage : Graduated cylinders are essential for preparing solutions with precise volumes and measuring liquids accurately.
- Purpose : Pipettes are used for precise measurement and transfer of small volumes of liquid. They come in various types, including micropipettes for ultra-precise measurements.
- Features : Pipettes have a calibrated scale for volume selection, and some are disposable while others are reusable and require calibration.
- Usage : Pipettes are commonly used in biology, chemistry, and analytical chemistry for tasks like transferring samples, making dilutions, and preparing standards.
- Purpose : Burets are used for precise titrations in analytical chemistry. They allow for controlled dispensing of a titrant into a solution.
- Features : Burets are long, graduated tubes with a stopcock at the bottom for controlling the flow of liquid.
- Usage : Burets are essential in titration experiments where the volume of titrant needed to reach a specific endpoint is critical.
Florence Flask
- Purpose : Florence flasks are used for boiling and heating liquids. They have a round bottom that allows for even heating.
- Features : They typically have a long neck and are often used with a rubber stopper or glass tubing for attaching other equipment.
- Usage : Florence flasks are commonly used in distillation setups and refluxing reactions.
- Purpose : Volumetric flasks are used for preparing solutions with precise volumes. They come in various sizes and are designed to hold a specific volume when filled to the calibration mark.
- Features : Volumetric flasks have a long neck with a single calibration mark on the neck.
- Usage : They are crucial for preparing accurate and known concentrations of solutions, such as standards used in chemical analysis.
- Purpose : Funnels are used for transferring liquids or fine-grained substances from one container to another. They help avoid spills and maintain accuracy.
- Features : Funnels have a wide, tapered opening at the top and a narrow spout at the bottom.
- Usage : Funnels are essential for tasks like filtering solutions, adding reagents to containers, and filling smaller vessels without spillage.
- Purpose : Crucibles are heat-resistant containers used for heating substances to high temperatures. They are typically made of porcelain or ceramic materials.
- Features : They have a small, cylindrical shape and come with lids.
- Usage : Crucibles are commonly used for processes such as heating samples to dryness, ashing organic materials, and performing high-temperature reactions.
- Purpose : Tongs are used for safely handling hot glassware and objects in the laboratory.
- Features : They have long, pincer-like arms with insulated handles.
- Usage : Tongs are essential for gripping and moving hot crucibles, beakers, flasks, and other equipment without direct contact.
Evaporating Dish
- Purpose : Evaporating dishes are shallow, flat-bottomed containers used for evaporating solvents from solutions.
- Features : They are typically made of porcelain or borosilicate glass and are resistant to high temperatures.
- Usage : Evaporating dishes are used to concentrate solutions by gently heating them to drive off the solvent, leaving behind the solute.
- Purpose : Desiccators are sealed containers used to store substances in a dry environment, protecting them from moisture.
- Features : They have an airtight seal and often contain a drying agent like silica gel or calcium chloride.
- Usage : Desiccators are used for storing moisture-sensitive materials, such as hygroscopic chemicals or humidity-sensitive samples.
- Purpose : Centrifuges are used for separating components of a liquid or mixture based on density by spinning them at high speeds.
- Features : They have a rotor that holds sample tubes and can generate centrifugal forces.
- Usage : Centrifuges are used in various fields, including biology, chemistry, and clinical laboratories, for tasks like separating cells, proteins, and particles from liquids.
- Purpose : A hot plate is an electric heating device used to heat glassware or other containers, usually with a flat, heated surface.
- Usage : Hot plates are commonly used for tasks such as boiling water, heating solutions, or conducting reactions that require controlled and consistent temperature.
Magnetic Stirrer
- Purpose : Magnetic stirrers use a rotating magnetic field to create a vortex in a liquid, which stirs or mixes the contents of a container without the need for a physical stirring rod.
- Usage : They are used for even and continuous mixing of solutions, particularly in chemistry and biology experiments.
- Purpose : A pH meter measures the acidity or alkalinity (pH) of a solution. It provides a numerical pH value based on the concentration of hydrogen ions in the solution.
- Usage : pH meters are vital in various fields, including chemistry, biology, and environmental science, for accurately determining pH levels in solutions.
- Purpose : A spectrophotometer measures the absorption or transmission of light by a substance across a range of wavelengths. It is used for quantitative analysis of substances in a solution.
- Usage : Spectrophotometers are essential for applications like quantifying the concentration of a solute, identifying compounds, and studying chemical reactions.
- Purpose : Autoclaves are pressurized and high-temperature chambers used to sterilize equipment, media, and samples in a laboratory.
- Usage : Autoclaves are crucial for maintaining sterile conditions in microbiology, biotechnology, and medical laboratories.
- Purpose : Incubators provide a controlled environment with regulated temperature and humidity for the growth of microorganisms or the incubation of biological samples.
- Usage : They are essential for cell culture, microbial culturing, and other biological research applications.
Refrigerator/Freezer
- Purpose : Laboratory refrigerators and freezers are used to store temperature-sensitive reagents, samples, and biological materials at controlled temperatures.
- Usage : They are crucial for preserving the integrity and stability of materials, such as enzymes, vaccines, and DNA.
- Purpose : A microcentrifuge is a high-speed centrifuge designed to spin small volumes of liquid at very high speeds, separating components based on density.
- Usage : They are used for tasks such as pelleting cells or particles, separating DNA, and isolating proteins.
Gel Electrophoresis Apparatus
- Purpose : Gel electrophoresis apparatus is used to separate and analyze DNA, RNA, or proteins based on their size and charge.
- Usage : It is a fundamental tool in molecular biology for tasks like DNA fingerprinting, DNA fragment separation, and protein analysis.
PCR Machine (Polymerase Chain Reaction)
- Purpose : A PCR machine amplifies specific DNA sequences through repeated cycles of heating and cooling.
- Usage : PCR machines are vital in molecular biology for DNA amplification, genetic testing, and DNA sequencing.
Spectrofluorometer
- Purpose : A spectrofluorometer measures the fluorescence emission spectra of substances when excited by light of a specific wavelength.
- Components : It typically includes a light source, monochromator, sample holder, and photodetector.
- Usage : Spectrofluorometers are used to study the fluorescence properties of compounds, such as fluorescent dyes, proteins, and biomolecules, in chemical and biological research. They are crucial for characterizing fluorescent materials and quantifying their concentrations.
Distillation Apparatus :
- Purpose : Distillation apparatus is used to separate components of a liquid mixture based on their different boiling points.
- Components : It comprises a boiling flask, distillation head, condenser, receiver flask, and a heat source.
- Usage : Distillation is a common technique for purifying or separating liquids in chemistry, including the production of distilled water or the isolation of pure chemicals.
Condenser :
- Purpose : A condenser cools and condenses vaporized substances back into a liquid state, typically in distillation setups.
- Components : It includes a coiled or straight glass tube through which cooling water circulates.
- Usage : Condensers are essential components in distillation and reflux processes, allowing the collection of purified liquids.
- Purpose : A spatula is a small, flat utensil used for transferring solid chemicals or powders.
- Materials : Spatulas are typically made of stainless steel, plastic, or glass.
- Usage : Spatulas are commonly used to weigh or transfer small quantities of solids in chemistry and analytical work. They come in various shapes and sizes to suit different applications.
Pipette Bulb :
- Purpose : A pipette bulb is a rubber bulb that attaches to a pipette for creating suction and facilitating liquid transfer.
- Usage : Pipette bulbs are used to draw liquids into pipettes accurately. They provide a manual means of controlling the volume of liquid aspirated and dispensed.
Buchner Funnel :
- Purpose : A Buchner funnel is used in vacuum filtration to separate solids from liquids. It contains a perforated plate and a vacuum source to pull liquid through.
- Components : It includes a funnel with a flat, porous base and a conical flask or vacuum flask below it.
- Usage : Buchner funnels are commonly used for isolating precipitates or collecting solid residues from liquid suspensions. Vacuum filtration speeds up the process.
Mortar and Pestle :
- Purpose : A mortar and pestle are tools used for grinding, crushing, and mixing solid materials into fine powders or pastes.
- Materials : Mortars are typically made of ceramic, glass, or stone, while the pestle is a heavy rod.
- Usage : They are widely used in chemistry and biology for tasks such as sample preparation, grinding chemicals, or creating homogenous mixtures.
Stirring Rod :
- Purpose : A stirring rod is a long, thin glass or plastic rod used for manually stirring liquids or suspensions.
- Usage : Stirring rods are commonly used for mixing solutions, ensuring homogeneity in reactions, and transferring small quantities of liquid.
Thermometer :
- Purpose : A thermometer measures temperature. Laboratory thermometers are designed for accuracy and precision.
- Types : There are various types of thermometers, including mercury-in-glass, digital, and infrared.
- Usage : Thermometers are used in various applications, from monitoring reaction temperatures to maintaining controlled conditions in incubators and ovens.
Melting Point Apparatus
- Purpose : A melting point apparatus is used to determine the melting point of a solid substance, which is a characteristic property.
- Components : It includes a heating block, sample holder, and a magnifying lens.
- Usage : It is employed in chemistry for identifying and verifying the purity of organic compounds by comparing their melting points to known standards.
- Purpose : A Petri dish is a shallow, flat, cylindrical container with a lid, used for culturing and observing microorganisms and small specimens.
- Materials : Petri dishes are typically made of glass or clear plastic.
- Usage : Petri dishes are widely used in microbiology for bacterial and fungal cultures and in various biological experiments, including bacterial plate counts and tissue culture.
Separatory Funnel
- Purpose : A separatory funnel is used to separate immiscible liquids or liquids with different densities.
- Components : It has a conical shape with a stopcock at the bottom for controlled liquid drainage.
- Usage : Separatory funnels are commonly used in chemistry for processes like liquid-liquid extraction, purification, and phase separations.
Gas Burette
- Purpose : A gas burette is a graduated glass tube used to measure the volume of gases in chemical experiments.
- Usage : It is employed in experiments where precise gas volume measurements are necessary, such as in gas collection or stoichiometry experiments.
Hemocytometer
- Purpose : A hemocytometer is a special counting chamber used for manually counting blood cells and other small particles under a microscope.
- Components : It consists of a thick glass slide with a grid etched on it and a coverslip.
- Usage : Hemocytometers are essential in clinical laboratories and research for accurate cell counting in applications like blood cell analysis and cell culture.
Vortex Mixer :
- Purpose : A vortex mixer is a high-speed mixer that creates a vortex in a liquid sample to mix its contents.
- Components : It has a motorized base with a rubber cup or platform for holding sample tubes.
- Usage : Vortex mixers are used to quickly and thoroughly mix liquids, suspensions, and small samples in test tubes or microcentrifuge tubes.
Ultrasonic Cleaner
- Purpose : An ultrasonic cleaner uses high-frequency sound waves to remove contaminants from objects immersed in a liquid.
- Components : It consists of a tank filled with cleaning solution, ultrasonic transducers, and a timer.
- Usage : Ultrasonic cleaners are commonly used to clean laboratory glassware, small parts, and delicate instruments, ensuring thorough cleaning without manual scrubbing.
TLC Plate (Thin-Layer Chromatography Plate)
- Purpose : TLC is a chromatography technique used to separate and analyze mixtures. A TLC plate is a flat, thin sheet coated with a stationary phase for this purpose.
- Components : The plate is typically made of glass or plastic with a thin layer of absorbent material (such as silica gel) as the stationary phase.
- Usage : Researchers spot or apply a sample mixture at the base of the plate, which is then placed in a solvent chamber. As the solvent rises through capillary action, it carries the components of the mixture, allowing for separation based on their interactions with the stationary phase.
Rotary Evaporator
- Purpose : A rotary evaporator is used for the gentle and efficient removal of solvents from liquid mixtures, typically in chemical synthesis or sample preparation.
- Components : It consists of a rotating flask, a water bath or heating bath, a vacuum system, and a condenser.
- Usage : The sample is placed in the rotating flask and heated under vacuum. The reduced pressure lowers the boiling point of the solvent, facilitating its removal. The condenser then collects the vapor, which condenses back into a liquid.
- Purpose : A viscometer measures the viscosity of a fluid, which is a measure of its resistance to flow.
- Types : There are various types of viscometers, including capillary viscometers, rotational viscometers, and falling ball viscometers.
- Usage : Viscometers are used in industries like pharmaceuticals, food, and oil to determine fluid properties and quality control. They are also employed in research to study the flow behavior of fluids.
- Purpose : A hydrometer is an instrument used to measure the specific gravity (density) of a liquid.
- Components : It typically consists of a graduated glass tube with a weighted bulb at the bottom.
- Usage : Hydrometers are commonly used in various applications, such as in breweries to measure the alcohol content of beer, in laboratories for density measurements, and in the petroleum industry for testing fuel quality.
Microtome :
- Purpose : A microtome is a precision instrument used to cut thin slices (sections) of biological or material samples for microscopy or analysis.
- Types : There are different types of microtomes, including rotary microtomes, cryostats, and ultramicrotomes.
- Usage : Microtomes are vital in histology, biology, and material science for preparing samples for examination under microscopes or other analytical instruments.
Autotitrator (Automatic Titrator)
- Purpose : An autotitrator is an automated titration instrument used for precise and efficient chemical analysis, especially in determining the concentration of analytes in a solution.
- Components : It consists of a burette, a titration vessel, a pH electrode, and automated control systems.
- Usage : Autotitrators perform titrations accurately and with reduced human error. They are widely used in analytical chemistry, quality control, and environmental monitoring.
Gas Syringe
- Purpose : A gas syringe is a device used to measure and transfer known volumes of gases in laboratory experiments.
- Components : It typically consists of a cylindrical glass tube with a plunger or piston.
- Usage : Gas syringes are used in experiments where precise gas volumes are required, such as in gas collection, gas stoichiometry, and determining gas properties like molar mass or density.
Specialized Laboratory Apparatus/Equipment
Nuclear Magnetic Resonance (NMR) Spectrometer :
- Purpose : An NMR spectrometer is used for the analysis of organic compounds’ structure and properties. It measures the nuclear magnetic resonance of atomic nuclei.
- Components : It consists of a powerful magnet, radiofrequency (RF) transmitter and receiver, and a sample holder.
- Usage : Researchers place a sample in the magnet, which aligns the nuclei with the magnetic field. RF pulses are applied, and the resulting signals provide information about the chemical environment and connectivity of atoms in the sample.
Scanning Electron Microscope (SEM)
- Purpose : SEM produces high-resolution images of the surface of specimens using focused electron beams.
- Components : It includes an electron source, electromagnetic lenses, a sample chamber, and detectors for secondary electrons and backscattered electrons.
- Usage : The electron beam scans the sample’s surface, and signals from interactions with the beam create detailed images, revealing surface topography and composition.
Gas Chromatography-Mass Spectrometry (GC-MS)
- Purpose : GC-MS combines gas chromatography with mass spectrometry to identify and quantify chemical compounds in a mixture.
- Components : It has a gas chromatograph to separate compounds and a mass spectrometer to analyze their masses.
- Usage : The mixture is vaporized and separated in the chromatograph. The separated compounds are then ionized in the mass spectrometer and identified by their mass-to-charge ratios.
High-Performance Liquid Chromatograph (HPLC)
- Purpose : HPLC separates and quantifies compounds in a liquid mixture based on their interactions with a stationary phase.
- Components : It includes a pump, injector, column, detector, and data system.
- Usage : Liquid samples are pumped through a column filled with stationary phase. Different compounds interact differently, leading to separation. The detector records signals that are used for quantification.
UV-Visible Spectrophotometer
- Purpose : This instrument measures the absorption of ultraviolet and visible light by a sample, often for quantitative analysis.
- Components : It has a light source, monochromator, sample holder, and detector.
- Usage : A beam of light passes through the sample, and the detector measures how much light is absorbed. This data can be used to determine the concentration of an absorbing substance.
Flame Photometer
- Purpose : Flame photometers are used to measure the concentration of specific elements in a sample by analyzing the color of the flame produced when the elements are atomized.
- Components : It consists of a flame, nebulizer, burner, and a system for detecting emitted light.
- Usage : A sample is introduced into the flame, and the characteristic colors produced are compared to known standards to determine the element’s concentration.
Mass Spectrometer
- Purpose : Mass spectrometers determine the molecular composition of a sample by measuring the mass-to-charge ratio of ions.
- Components : They include an ionization source, mass analyzer, and detector.
- Usage : Samples are ionized, and the resulting ions are separated based on their mass-to-charge ratio. The detector records these ions, providing information about the sample’s composition.
Atomic Force Microscope (AFM)
- Purpose : AFMs allow for imaging and manipulating materials at the nanoscale by scanning a sharp tip across the surface.
- Components : AFMs have a cantilever with a sharp tip and a detector for measuring tip-sample interactions.
- Usage : The tip is brought close to the sample’s surface, and interactions between the tip and sample are measured, producing high-resolution topographical images.
Differential Scanning Calorimeter (DSC)
- Purpose : DSC measures changes in heat flow associated with phase transitions and chemical reactions in materials.
- Components : It consists of a sample holder, reference cell, and heating element.
- Usage : The sample and a reference are heated or cooled simultaneously, and the heat flow difference between them is recorded. This provides information about thermal properties and transitions.
Gas Density Meter
- Purpose : Gas density meters determine the density of gases under varying conditions of temperature and pressure.
- Components : They typically involve a sensor that measures the speed of sound in the gas.
- Usage : By measuring the speed of sound, these meters can calculate the density of gases, which is important in various industrial and research applications.
Circular Dichroism Spectrometer (CD)
- Purpose : CD spectrometers analyze the optical activity of chiral molecules to determine their secondary structure.
- Components : They include a light source, sample holder, and detectors for measuring differences in left and right circularly polarized light.
- Usage : CD spectroscopy is widely used in chemistry and biochemistry to study the conformation of biomolecules like proteins and nucleic acids.
- Purpose : Ultracentrifuges separate particles in suspensions based on size and density using high centrifugal forces.
- Components : They have a rotor, sample tubes, and a powerful motor for high-speed spinning.
- Usage : Ultracentrifugation is essential for tasks like separating macromolecules, organelles, or colloidal particles in biological and biochemical research.
Sonication Bath
- Purpose : Sonication baths use high-frequency sound waves to disrupt and disperse particles in liquids for sample preparation.
- Components : They consist of a bath filled with liquid and a sonication probe or transducer.
- Usage : Sonication is employed for tasks like cell disruption, homogenization, and degassing of solutions.
Raman Spectrometer
- Purpose : Raman spectrometers measure the scattering of monochromatic light by molecules to identify and characterize chemical compounds.
- Components : They include a laser source, spectrometer, and a detector for Raman scattering.
- Usage : Raman spectroscopy is used for chemical analysis, materials characterization, and identifying molecular structures.
Atomic Emission Spectrometer
- Purpose : Atomic emission spectrometers analyze the emission of light by excited atoms to determine elemental composition in samples.
- Components : They include a sample introduction system, excitation source (flame or plasma), and a detector.
- Usage : This instrument is widely used in elemental analysis, such as in environmental monitoring and metal analysis.
Microplate Reader
- Purpose : Microplate readers read absorbance, fluorescence, or luminescence in microplate wells for high-throughput screening and assays.
- Components : They have multiple detectors and can accommodate microplates with multiple sample wells.
- Usage : Microplate readers are essential in molecular biology, biochemistry, and drug discovery for rapid analysis of numerous samples.
Chromatography Data System (CDS)
- Purpose : A Chromatography Data System is software used to control and analyze data from chromatography instruments.
- Components : It includes data acquisition, processing, and reporting capabilities.
- Usage : CDS is crucial for managing and interpreting data generated from chromatography experiments, ensuring accurate and reliable results.
Cryo-Electron Microscope
- Purpose : Cryo-EM uses extremely low temperatures to study the structure of biological macromolecules and large assemblies.
- Components : It includes a specialized electron microscope and a cryogenic sample stage.
- Usage : Cryo-EM is revolutionizing structural biology by enabling the visualization of complex structures at near-atomic resolution.
Potentiostat-Galvanostat
- Purpose : A potentiostat-galvanostat is used to control and measure electrochemical reactions, often in corrosion studies and battery research.
- Components : It has three electrodes (working, reference, and counter electrodes) and a control unit.
- Usage : It’s employed in a wide range of electrochemical experiments, including corrosion rate determination and battery testing.
Laser Ablation-Inductively Coupled Plasma-Mass Spectrometer (LA-ICP-MS) :
- Purpose : LA-ICP-MS analyzes solid samples by vaporizing them with a laser and measuring the elemental composition with ICP-MS.
- Components : It involves a laser ablation system coupled to an ICP-MS instrument.
- Usage : LA-ICP-MS is used for spatially-resolved elemental analysis in various fields, including geology, environmental science, and materials research.
Further References
- Laboratory Apparatus : https://owlcation.com/stem/A-Chemistry-Guide-List-of-Common-Laboratory-Equipment-Names-and-Uses
- Lab Equipments : https://www.google.com/amp/s/www.cnlabglassware.com/more-than-20-common-laboratory-apparatus-their-uses.html%3famp=1?espv=1
Related posts:
- Polyphosphoric Acid (H3PO4): Properties And Uses
- Tungsten(W): Properties, Use, Health & Environmental Effects
- Bunsen Burner Parts: Operation, Uses And Flames
- Cyclopentanol or Cyclopentyl alcohol: Preparation and Properties
- Different Types Of Electrodes
- Bismuth (Bi): Element Information, Properties And Uses
Advertisement
10 Scientific Laws and Theories You Really Should Know
- Share Content on Facebook
- Share Content on LinkedIn
- Share Content on Flipboard
- Share Content on Reddit
- Share Content via Email
Scientists have many tools available to them when attempting to describe how nature and the universe at large work. Often they reach for laws and theories first. What's the difference? A scientific law can often be reduced to a mathematical statement, such as E = mc²; it's a specific statement based on empirical data, and its truth is generally confined to a certain set of conditions. For example, in the case of E = mc², c refers to the speed of light in a vacuum.
A scientific theory often seeks to synthesize a body of evidence or observations of particular phenomena. It's generally — though by no means always — a grander, testable statement about how nature operates. You can't necessarily reduce a scientific theory to a pithy statement or equation, but it does represent something fundamental about how nature works.
Both laws and theories depend on basic elements of the scientific method, such as generating a hypothesis , testing that premise, finding (or not finding) empirical evidence and coming up with conclusions. Eventually, other scientists must be able to replicate the results if the experiment is destined to become the basis for a widely accepted law or theory.
In this article, we'll look at 10 scientific laws and theories that you might want to brush up on, even if you don't find yourself, say, operating a scanning electron microscope all that frequently. We'll start off with a bang and move on to the basic laws of the universe, before hitting evolution . Finally, we'll tackle some headier material, delving into the realm of quantum physics.
- Big Bang Theory
- Hubble's Law of Cosmic Expansion
- Kepler's Laws of Planetary Motion
- Universal Law of Gravitation
- Newton's Laws of Motion
- Laws of Thermodynamics
- Archimedes' Buoyancy Principle
- Evolution and Natural Selection
- Theory of General Relativity
- Heisenberg's Uncertainty Principle
10: Big Bang Theory
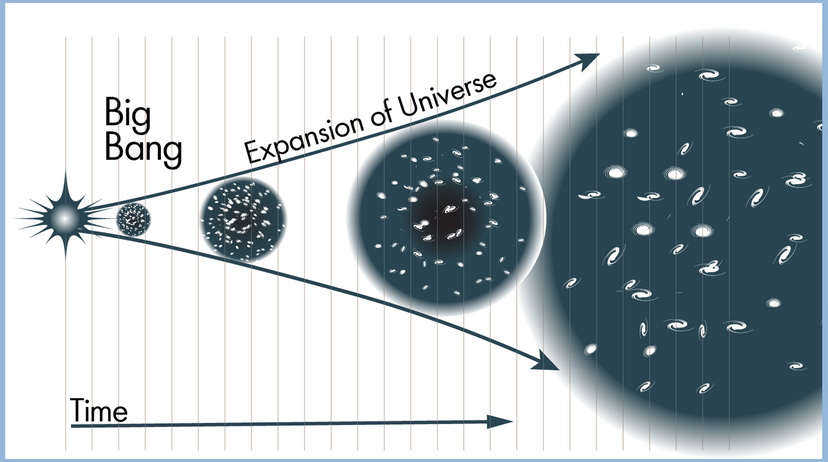
If you're going to know one scientific theory, make it the one that explains how the universe arrived at its present state. Based on research performed by Edwin Hubble, Georges Lemaitre and Albert Einstein, among others, the big bang theory postulates that the universe began almost 14 billion years ago with a massive expansion event. At the time, the universe was confined to a single point, encompassing all of the universe's matter. That original movement continues today, as the universe keeps expanding outward.
The theory of the big bang gained widespread support in the scientific community after Arno Penzias and Robert Wilson discovered cosmic microwave background radiation in 1965. Using radio telescopes, the two astronomers detected cosmic noise, or static, that didn't dissipate over time. Collaborating with Princeton researcher Robert Dicke, the pair confirmed Dicke's hypothesis that the original big bang left behind low-level radiation detectable throughout the universe.
9: Hubble's Law of Cosmic Expansion
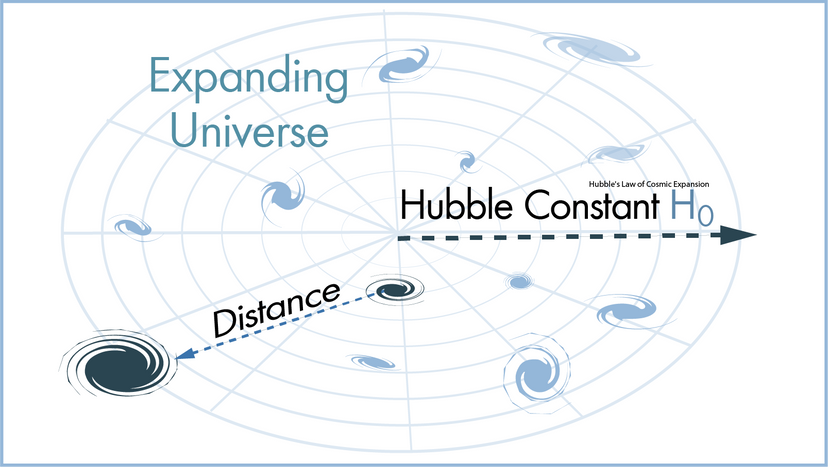
Let's stick with Edwin Hubble for a second. While the 1920s roared past and the Great Depression limped by, Hubble was performing groundbreaking astronomical research. Hubble not only proved that there were other galaxies besides the Milky Way , he also discovered that these galaxies were zipping away from our own, a motion he called recession .
In order to quantify the velocity of this galactic movement, Hubble proposed Hubble's Law of Cosmic Expansion , aka Hubble's law, an equation that states: velocity = H × distance . Velocity represents the galaxy's recessional velocity; H is the Hubble constant, or parameter that indicates the rate at which the universe is expanding; and distance is the galaxy's distance from the one with which it's being compared.
Hubble's constant has been calculated at different values over time, but the current accepted value is 70 kilometers/second per megaparsec, the latter being a unit of distance in intergalactic space [source: White ]. For our purposes, that's not so important. What matters most is that Hubble's law provides a concise method for measuring a galaxy's velocity in relation to our own. And perhaps most significantly, the law established that the universe is made up of many galaxies, whose movements trace back to the big bang.
8: Kepler's Laws of Planetary Motion
For centuries, scientists battled with one another and with religious leaders about the planets' orbits, especially about whether they orbited our sun. In the 16th century, Copernicus put forth his controversial concept of a heliocentric solar system, in which the planets revolved around the sun — not Earth. But it would take Johannes Kepler, building on work performed by Tyco Brahe and others, to establish a clear scientific foundation for the planets' movements.
Kepler's three laws of planetary motion — formed in the early 17th century — describe how planets orbit the sun. The first law, sometimes called the law of orbits , states that planets orbit the sun elliptically. The second law, the law of areas , states that a line connecting a planet to the sun covers an equal area over equal periods of time. In other words, if you're measuring the area created by drawing a line from Earth to the sun and tracking Earth's movement over 30 days, the area will be the same no matter where Earth is in its orbit when measurements begin.
The third one, the law of periods , allows us to establish a clear relationship between a planet's orbital period and its distance from the sun. Thanks to this law, we know that a planet relatively close to the sun, like Venus, has a far briefer orbital period than a distant planet, such as Neptune.
7: Universal Law of Gravitation
We may take it for granted now, but more than 300 years ago Sir Isaac Newton proposed a revolutionary idea: that any two objects, no matter their mass, exert gravitational force toward one another. This law is represented by an equation that many high schoolers encounter in physics class. It goes as follows:
F = G × [(m 1 m 2 )/r 2 ]
F is the gravitational force between the two objects, measured in Newtons. M 1 and m 2 are the masses of the two objects, while r is the distance between them. G is the gravitational constant , a number currently calculated to be 6.672 × 10 -11 N m 2 kg -2 [source: Weisstein ].
The benefit of the universal law of gravitation is that it allows us to calculate the gravitational pull between any two objects. This ability is especially useful when scientists are, say, planning to put a satellite in orbit or charting the course of the moon .
6: Newton's Laws of Motion
As long as we're talking about one of the greatest scientists who ever lived, let's move on to Newton's other famous laws. His three laws of motion form an essential component of modern physics. And like many scientific laws, they're rather elegant in their simplicity.
The first of the three laws states an object in motion stays in motion unless acted upon by an outside force. For a ball rolling across the floor, that outside force could be the friction between the ball and the floor, or it could be the toddler that kicks the ball in another direction.
The second law establishes a connection between an object's mass ( m ) and its acceleration ( a ), in the form of the equation F = m × a . F represents force, measured in Newtons. It's also a vector, meaning it has a directional component. Owing to its acceleration, that ball rolling across the floor has a particular vector , a direction in which it's traveling, and it's accounted for in calculating its force.
The third law is rather pithy and should be familiar to you: For every action there is an equal and opposite reaction. That is, for every force applied to an object or surface, that object pushes back with equal force.
5: Laws of Thermodynamics
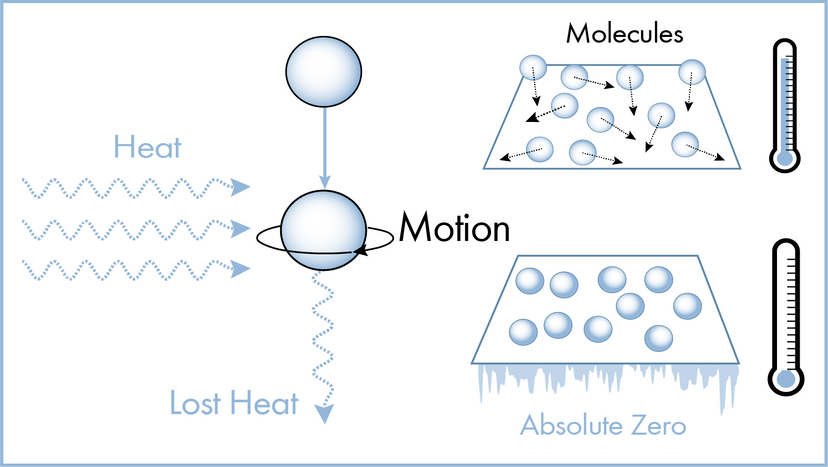
The British physicist and novelist C.P. Snow once said that a nonscientist who didn't know the second law of thermodynamics was like a scientist who had never read Shakespeare [source: Lambert]. Snow's now-famous statement was meant to emphasize both the importance of thermodynamics and the necessity for nonscientists to learn about it.
Thermodynamics is the study of how energy works in a system, whether it's an engine or Earth's core. It can be reduced to several basic laws, which Snow cleverly summed up as follows [source: Physics Planet]:
- You can't win.
- You can't break even.
- You can't quit the game.
Let's unpack these a bit. By saying you can't win, Snow meant that since matter and energy are conserved, you can't get one without giving up some of the other (i.e., E=mc²). It also means that for an engine to produce work, you have to supply heat, although in anything other than a perfectly closed system, some heat is inevitably lost to the outside world, which then leads to the second law.
The second statement — you can't break even — means that due to ever-increasing entropy , you can't return to the same energy state. Energy concentrated in one place will always flow to places of lower concentration.
Finally, the third law — you can't quit the game — refers to absolute zero, the lowest theoretical temperature possible, measured at zero Kelvin or (minus 273.15 degrees Celsius and minus 459.67 degrees Fahrenheit). When a system reaches absolute zero, molecules stop all movement, meaning that there is no kinetic energy, and entropy reaches its lowest possible value. But in the real world, even in the recesses of space, reaching absolutely zero is impossible — you can only get very close to it.
4: Archimedes' Buoyancy Principle
After he discovered his principle of buoyancy, the ancient Greek scholar Archimedes allegedly yelled out "Eureka!" and ran naked through the city of Syracuse. The discovery was that important. The story goes that Archimedes made his great breakthrough when he noticed the water rise as he got into the tub [source: Quake ].
According to Archimedes' buoyancy principle , the force acting on, or buoying, a submerged or partially submerged object equals the weight of the liquid that the object displaces. This sort of principle has an immense range of applications and is essential to calculations of density, as well as designing submarines and other oceangoing vessels.
3: Evolution and Natural Selection
Now that we've established some of the fundamental concepts of how our universe began and how physics play out in our daily lives, let's turn our attention to the human form and how we got to be the way we are. According to most scientists, all life on Earth has a common ancestor. But in order to produce the immense amount of difference among all living organisms, certain ones had to evolve into distinct species.
In a basic sense, this differentiation occurred through evolution, through descent with modification [source: UCMP ]. Populations of organisms developed different traits, through mechanisms such as mutation. Those with traits that were more beneficial to survival such as, a frog whose brown coloring allows it to be camouflaged in a swamp, were naturally selected for survival; hence the term natural selection .
It's possible to expand upon both of these theories at greater length, but this is the basic, and groundbreaking, discovery that Darwin made in the 19th century: that evolution through natural selection accounts for the tremendous diversity of life on Earth.
2: Theory of General Relativity
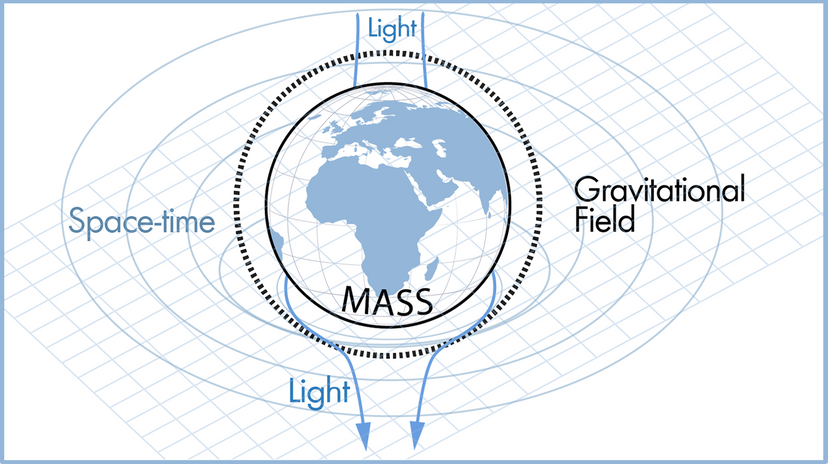
Albert Einstein's theory of general relativity remains an important and essential discovery because it permanently altered how we look at the universe. Einstein's major breakthrough was to say that space and time are not absolutes and that gravity is not simply a force applied to an object or mass. Rather, the gravity associated with any mass curves the very space and time (often called space-time) around it.
To conceptualize this, imagine you're traveling across the Earth in a straight line, heading east, starting somewhere in the Northern Hemisphere. After a while, if someone were to pinpoint your position on a map, you'd actually be both east and far south of your original position. That's because Earth is curved. To travel directly east, you'd have to take into account the shape of Earth and angle yourself slightly north. (Think about the difference between a flat paper map and a spherical globe.)
Space is pretty much the same. For example, to the occupants of the shuttle orbiting Earth, it can look like they're traveling on a straight line through space. In reality, the space-time around them is being curved by Earth's gravity (as it would be with any large object with immense gravity such as a planet or a black hole), causing them to both move forward and to appear to orbit Earth.
Einstein's theory had tremendous implications for the future of astrophysics and cosmology. It explained a minor, unexpected anomaly in Mercury's orbit, showed how starlight bends and laid the theoretical foundations for black holes.
1: Heisenberg's Uncertainty Principle
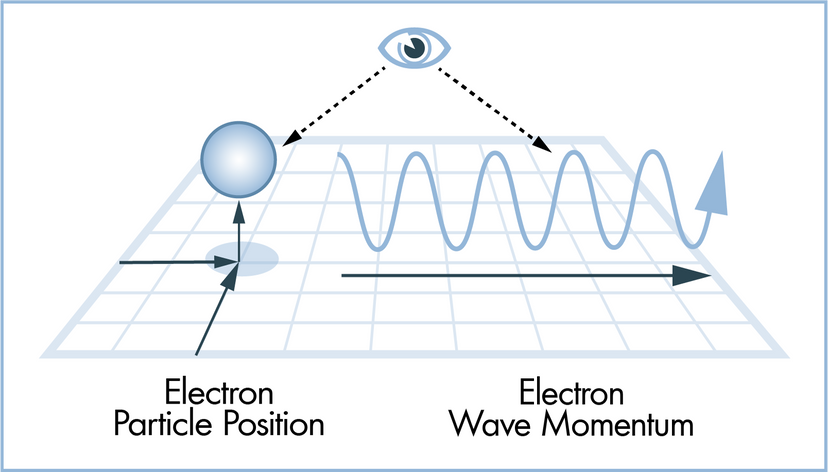
Einstein's broader theory of relativity told us more about how the universe works and helped to lay the foundation for quantum physics, but it also introduced more confusion into theoretical science. In 1927, this sense that the universe's laws were, in some contexts, flexible, led to a groundbreaking discovery by the German scientist Werner Heisenberg.
In postulating his Uncertainty Principle , Heisenberg realized that it was impossible to simultaneously know, with a high level of precision, two properties of a particle. In other words, you can know the position of an electron with a high degree of certainty, but not its momentum and vice versa.
Niels Bohr later made a discovery that helps to explain Heisenberg's principle. Bohr found that an electron has the qualities of both a particle and a wave, a concept known as wave-particle duality , which has become a cornerstone of quantum physics. So when we measure an electron's position, we are treating it as a particle at a specific point in space, with an uncertain wavelength. When we measure its momentum, we are treating it as a wave, meaning we can know the amplitude of its wavelength but not its location.
Keep reading for more science stuff you might like.
Scientific Theory FAQ
What is scientific theory, what is an example of scientific theory, is a scientific law more accurate than a scientific theory, what are the five scientific laws, lots more information, related articles.
- Gravitational Waves! Or the Chirps That Prove Einstein Was Right
- How Newton's Laws of Motion Work
- 10 Scientific Words You're Probably Using Wrong
- How the Scientific Method Works
- Ask an Astronomer. "The Theory of Relativity." Cornell University Astronomy Dept. March 21, 2008. (Jan. 5, 2011) http://curious.astro.cornell.edu/relativity.php
- Bragg, Melvyn. "The Second Law of Thermodynamics." BBC. Dec. 16, 2004. (Jan. 5, 2011) http://www.bbc.co.uk/programmes/p004y2bm
- Glenn Research Center. "First Law of Thermodynamics." NASA. July 11, 2008. (Jan. 5, 2011) http://www.grc.nasa.gov/WWW/K-12/airplane/thermo1.html
- Lambert, Frank L. "Shakespeare and Thermodynamics: Dam the Second Law!" Occidental College. 2008. (Jan. 5, 2011) http://shakespeare2ndlaw.oxy.edu/
- LaRocco, Chris and Blair Rothstein. "The Big Bang." University of Michigan. (Jan. 5, 2011) http://www.umich.edu/~gs265/bigbang.htm
- Lightman, Alan. "Relativity and the Cosmos." PBS Nova. June 2005. (Jan. 5, 2011) http://www.pbs.org/wgbh/nova/einstein/relativity/
- Matson, Ronald H. "Scientific Laws and Theories." Kennesaw State University. (Jan. 5, 2011) http://science.kennesaw.edu/~rmatson/3380theory.html
- Nave, C.R. "Hubble law and the expanding universe." Georgia State University. (Jan. 5, 2011) http://hyperphysics.phy-astr.gsu.edu/hbase/astro/hubble.html
- Nave, C.R. "Kepler's Laws." Georgia State University. (Jan. 5, 2011) http://hyperphysics.phy-astr.gsu.edu/hbase/kepler.html
- Nave, C.R. "The Uncertainty Principle." Georgia State University. (Jan. 5, 2011) http://hyperphysics.phy-astr.gsu.edu/hbase/uncer.html
- PBS. "Big bang theory is introduced." 1998. (Jan. 5, 2011) http://www.pbs.org/wgbh/aso/databank/entries/dp27bi.html
- PBS. "Heisenberg states the uncertainty principle." 1998. (Jan. 5, 2011) http://www.pbs.org/wgbh/aso/databank/entries/dp27un.html
- PBS. "Penzias and Wilson discover cosmic microwave radiation." 1998. (Jan. 5, 2011) http://www.pbs.org/wgbh/aso/databank/entries/dp65co.html
- Pidwirny, Michael. "Laws of Thermodynamics." Physical Geography. April 6, 2010. (Jan. 5, 2011) http://www.physicalgeography.net/fundamentals/6e.html
- Quake, Stephen. "Practically pure." The New York Times. Nov. 8, 2009. (Jan. 5, 2011) http://www.nytimes.com/2009/02/18/opinion/18iht-edquake.1.20274600.html
- Stern, David P. "Kepler's Three Laws of Planetary Motion." Phy6.org. March 21, 2005. (Jan. 5, 2011) http://www.phy6.org/stargaze/Kep3laws.htm
- Stern, David P. "Newton's theory of 'Universal Gravitation'." NASA. March 24, 2006. (Jan. 5, 2011) http://www-istp.gsfc.nasa.gov/stargaze/Sgravity.htm
- University of California Museum of Paleontology (UCMP). "Understanding Evolution: An introduction to evolution." (Jan. 5, 2011) http://evolution.berkeley.edu/evolibrary/article/0_0_0/evo_02
- University of California Museum of Paleontology (UCMP). "Understanding Evolution: Natural selection." (Jan. 5, 2011) http://evolution.berkeley.edu/evolibrary/article/evo_25
- University of Tennessee, Knoxville, Dept. of Physics & Astronomy. "Newton's Three Laws of Motion." (Jan. 5, 2011) http://csep10.phys.utk.edu/astr161/lect/history/newton3laws.html
- University of Tennessee, Knoxville, Dept. of Physics & Astronomy. "Sir Isaac Newton: The Universal Law of Gravitation." (Jan. 5, 2011) http://csep10.phys.utk.edu/astr161/lect/history/newtongrav.html
- Weisstein, Eric W. "Gravitational Constant." Wolfram Research. (Jan. 5, 2011) http://scienceworld.wolfram.com/physics/GravitationalConstant.html
- Weisstein, Eric W. "Kepler's Laws." Wolfram Research. (Jan. 5, 2011)http://scienceworld.wolfram.com/physics/KeplersLaws.html
- White, Martin. "The Hubble Expansion." University of California, Berkeley. (Jan. 5, 2011) http://astro.berkeley.edu/~mwhite/darkmatter/hubble.html
Please copy/paste the following text to properly cite this HowStuffWorks.com article:

100 Science Experiments for Kids that Use Materials You Already Own!
One of the best ways to inspire a love of science and learning in your kids is to introduce them to science experiments for kids at an early age.
These science experiment ideas are all simple enough for elementary kids and are designed for kids in elementary school and are a good place to start when just starting out trying STEM activities for kids !
However, these science project ideas are easy enough to expand into something that would also work as a middle school science experiment and many of them can also be simplified for kids in kindergarten, preschool, and even for toddlers!

It’s never too early to start science experiments with kids.
Easy and Low-Cost Science Experiments for Kids
Try these easy and fun science experiments when you need a no-fuss science experiment for kids! You can’t fail with these science experiments for kids!
If this is one of your first times doing a science experiment, or teaching one, you’ll want to brush up on my guide on how to do science experiments . It’s not just about the flashy reaction!
Over 100 Low-Prep Science Experiments and Lesson Plans!
We love science, and this list of science projects will keep you busy with science activities for years! Did you know that the benefits of hands-on science experiments are scientifically documented? You can read all about the benefits of science experiments here.
And if you’re wondering, “Hey, what is a science experiment anyway?” then you can read all about that, too!
If you’re getting started with science, try these easy science experiments that even non-science teachers can easily do with kids.
If you’re more familiar with science, keep scrolling to get to the list of 100s of science experiments with dozens of themes!

Science Supplies for Teachers
If you are new to teaching science, you might not know exactly what supplies you’ll need throughout the year. If you don’t know where to start, check out my list of must-have supplies for science experiments, or you can browse the lists below to start shopping right away!
Elementary Science Kits for Kids
Here are some of our favorite science kits to do in the classroom as a group in elementary!

STEM Kits for 1st Grade
If you love science and STEM but don’t like hunting for supplies, we love the Mel Science kits. They have options for elementary and middle school, and each kit comes with all the supplies needed for each experiment!
Print a lot of papers? Get $10 your HP Instant Ink order when you use this link.
Science Experiment Topics from Geology to Biology
If you want to study a specific science topic, these science experiments are the place to start! From geology to weather, science is tons of fun when you pick the right topic!
Here is the list of my favorite elementary science topics. There are so many fun science themes and topics to chose from.

Easy Season Experiments to Make Science Fun Year-Round
Try these fun season experiments with seasonal twists for winter, spring, summer, and fall- and do science all year long! You can have fun with the season and learn with science all at the same time!
Here are the best winter science experiments perfect for kids of all ages.
When spring is around the corner, try these spring science experiments!
During the summer, don’t let science learning slip! Instead, do these summer science experiments .
And this fall, try some of the fun fall science experiments on this list.
Science Experiments by Grade
These experiments are curated with ages and grade levels of children in mind. There is so much science to learn at every stage and in every grade!
For the youngest scientist, try these preschool science experiments. Easy science projects will start them off right with a deep love of science from the start!
Science Fair Project Ideas
Entering a science fair? Here you’ll find all the science fair project ideas you need!
And here are some elementary science fair projects designed for kids in 2nd to 6th grade.
More Fun Science Themes for Kids
There are tons of fun science themes for kids that go beyond a science topic or season of the year. If you are looking for out-of-the-box science themes, then you’ll love this list of creative science themes for kids!
Get the list of our favorite science themes for kids right here! Find experiment ideas from mermaids and carnivals to gross science and candy science! You really can help any child fall in love with science with these create science theme ideas.
Share this project with a friend!
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- MEMBER LOGIN
Hands On As We Grow®
Hands on kids activities for hands on moms. Focusing on kids activities perfect for toddlers and preschoolers.
50 Amazingly Simple Science Experiments for Kids at Home
Science Kindergartners Preschoolers Experiment Resources 31 Comments
Kids love experimenting , and these 50 simple science experiments for kids at home from Brigitte are perfect for all ages! Plus, you probably already have the basic supplies at home.
My daughters and I have had a lot of fun doing science experiments. Each year when we create our spring and summer list , we make sure to include “science days” which are days filled with science experiments.
Sometimes our science experiments don’t work according to plan, but I have been told that all scientists have failures with experiments from time to time.
It’s okay if they aren’t all successes.
Get the FREE Science Experiments Download
50 Simple Science Experiments with Supplies You Already Have

I love these 50 simple science experiments for you to try with your little scientists. They all use basic household supplies that you probably already have at home!
Most of these are experiments my daughters and I have done together. I hope you enjoy them as much as we have!
Get little ones involved with these easy toddler-friendly science experiment ideas!

Simple Science Experiments with Water
Not only can water be a blast to play in, but water plus a few basic supplies equals a lot of science fun!
- Make an orange sink and float with an orange buoyancy experiment from Playdough to Plato.
- Compare the amount of salt in different types of water with this salty egg experiment as seen on Uplifting Mayhem.
- Do a little more sinking or floating with a fun sink or float experiment even toddlers can do from Hands On As We Grow.
- Use the free printable to record what sinks or floats in an outdoor experiment from Buggy and Buddy.
- Create some beautiful pieces of paper with this rainbow paper experiment from Science Kiddo.
- Talk about solutions as you try the “what dissolves in water” experiment as seen on Hands On As We Grow.
- Learn about water absorption with this simple experiment from Little Bins for Little Hands.
- Mix some fun colors with this oil and water experiment from Fun Learning for Kids.
- Make your own lava lamp , just like on Hands On As We Grow.
- Can you keep all the water in the bag? Try it with a leak-proof bag experiment as seen on Hands On As We Grow.
- Learn about surface tension with this magic finger pepper experiment found on Hands On As We Grow.
- Make your own water cycle in a bottle as seen on A Dab of Glue Will Do.

Simple Science Experiments with Baking Soda and Vinegar
Baking soda + vinegar = a great chemical reaction! This fizzy reaction can fuel a variety of simple science experiments at home.
First of all, we have tested and found out the absolute best combination of baking soda and vinegar to get the best reaction possible. It makes a difference if you add vinegar to baking soda or vice versa! And how much you use!
- Inflate a balloon without blowing into it with a baking soda and vinegar balloon experiment as seen on Little Bins for Little Hands.
- Practice colors as you do a baking soda and vinegar with color experiment as seen on Hands On As We Grow.
- Have fun outside with an outdoor volcano eruption as seen on Preschool Inspirations.
- Have more volcano fun by making apple volcanoes as seen on The Resourceful Mama.
- Learn about acids and bases and the chemical reaction that occurs when you make apple seeds dance with a jumping apple seeds experiment as seen on JDaniel4s Mom.
- Watch some rice dance with a dancing rice experiment as seen on Green Kid Crafts.
- Continue your dance party by making raisins dance with a dancing raisin experiment as seen on 123 Homeschool 4 Me. What other items can you get to dance?
- Learn more about acids and bases by dissolving a sea shell as seen on Teach Beside Me.
- Make an egg shell disappear with this disappearing egg activity as seen on Premeditated Leftovers.
- See how far you can launch a soda bottle with this baking soda powered boat as seen on Science Sparks.
- Make your own rocks (or eggs) with this fizzy treasure rocks experiment as seen on Living Life and Learning.
- Have some fun this summer with this frozen vinegar experiment as seen on Inspiration Laboratories.
Plant Themed Simple Science Experiments
Enjoy learning about seeds, plant parts, and how plants grow with these simple science experiments.
- Learn about how plants soak up water through their stems with a flower experiment for kids from Growing A Jeweled Rose.
- Watch seeds sprout as you grow seeds in a jar as seen on Teaching Mama.
- Learn about the parts of the seed with a seed coat experiment as seen on Gift of Curiosity.
- Build a house out of sponges and then watch it sprout with this sprout house as seen on The Stem Laboratory.
- Learn what liquids allow seeds to grow the best with this seed experiment as seen on Gift of Curiosity.
- Explore how plants grow towards the light with this shoe-box maze experiment from Plants for Kids.

Animal Themed Simple Science Experiments
Learning about animals can be even more fun with some simple hands-on simple science experiments.
- Find out more about giraffes and create some giraffe spots as seen on Preschool Powol Packets.
- Learn about how animals in the Arctic keep warm by making an arctic glove as seen on Steve Spangler Science.
- Discover how penguins stay dry with a penguin feather experiment as seen on Raising Little Superheroes.
- Learn about different bird beaks with a bird beak experiment as seen on Blessed Beyond a Doubt.
- Explore how fish (and hermit crabs) breathe with this gill experiment as seen on Preschool Powol Packets.
- Learn about sharks with a shark buoyancy experiment as seen on Little Bins for Little Hands.
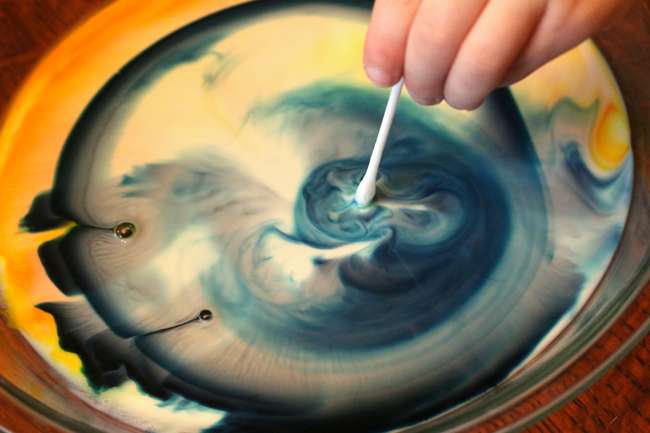
Even More Simple Science Experiment for Kids at Home!
If you are still looking for more science fun, you may enjoy the following simple science experiments.
- Find out how sugary drinks hurt teeth with an eggs-periment as seen on Feels Like Home Blog.
- Discover geodes (the state rock of Iowa) with this eggshell geode crystal experiment as seen on Science Bob.
- Learn about air pressure with an egg and bottle experiment as seen on Science Sparks.
- Find out what causes an apple to brown with this apple science experiment as seen on Teach Beside Me.
- Make an edible bubble apple with an experiment as seen on Preschool Powol Packet.
- Learn more about surface tension with a penny and water experiment as seen on Artful Parent.
- Mix colors like magic with this color changing milk experiment from Hands On As We Grow.
- Blow up a balloon with this soda and balloon experiment from Learn Play Imagine.
- Practice letters by making beautiful crystal letters as seen on Books and Giggles.
- Make your own indoor hovercraft as seen on Living Life and Learning.
- Learn about colors with this beautiful butterfly chromatography craft as seen on Buggy and Buddy.
- Make soap souffle as seen on Steve Spangler Science.
- After talking about liquids and solids (and finding them in your own home), create oobleck as seen on Babble Dabble Do. Is it a liquid, or is it a solid?
- Learn about frost by making some indoor frost as seen on Little Bin for Little Hands.
- Make your own homemade butter in a jar as seen on Happy Hooligans.
What scientific experiment will you try first?

About Brigitte Brulz
Brigitte Brulz is a homeschooling mom of two daughters, wife of her high school sweetheart, and author of Jobs of a Preschooler and Pickles, Pickles, I Like Pickles. She offers free coloring pages and activity ideas on her website at BrigitteBrulz.com .
More Hands on Kids Activities to Try

Reader Interactions
31 comments.
y2matewebsite says
October 2, 2024 at 2:39 am
I love this list of experiments! It’s amazing how many fun and educational activities we can do with everyday supplies. Can’t wait to try the vinegar and baking soda volcano with my kids this weekend! Thank you for sharing!
college brawl says
March 13, 2024 at 1:05 am
Wow, these experiments look like so much fun! I can’t wait to try them out with my kids. We’re always looking for new and creative ways to learn about science at home, and these experiments look like they’ll be perfect for us. Thanks for sharing! 😊
threadsBay says
August 31, 2023 at 3:13 am
I love science experiments! This one is really simple and easy to do.
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .

What Parents Have to Say…
- Hands On As We Grow Newsletter "It is hard to think of things to keep the 3 year old entertained and engaged while taking care of the baby. Everything we have tried so far from your website, the three year old has loved. Your ideas are so simple and he can do them for hours. HEAVEN!" - Karen I.
- I am now one happy Dad! "You have changed how I spend time with my kid in so many positive ways! I am now one happy dad that no longer wonders what I am going to do with this little guy for the next 12 hours :P Your site was this first time dad life saver!" - Jack C.
- Hands On As We Grow Newsletter "I feel like a new mama having so many fun ideas. I used to dread the afternoon, after naps, as it was so boring doing the same thing day after day but now I look forward to our "play" time!" - Haley S.
- Free Activity Challenge "Thank you so much for these activities. They have proven to me that I CAN be that mom that does cool and creative things with her kids! And those cool and creative things can actually be quite simple! What a revelation. Thank you!!" - Katie M.
- Activity Plans eBooks "This takes down the need to scour the internet for ideas. It's like looking for a recipe on the internet, so many options that often times it is less overwhelming to look in a book on the shelf than stress about too many options." Robyn G
- So organized and easy to use. I have found it impossible to Google ideas from a million different sites, get organized, shop for supplies, etc. This is exactly what I have been searching for! Thanks for making something so organized and easy to use. - Early Years Activity Plans User, Melissa C.
- The Activity Room Member "I absolutely love that this takes all the prep work out of engaging my children. It is so easy to just put up the calendar, and glance at it for inspiration when we are in a funk." - Rachel
- Grateful to have activities handed to us... "There's always something new for us to discover, as well as old favorites. I am so grateful to have activities handed to us... Fun and easy ones that can be put together in a moment's notice! You're awesome Jamie and I appreciate you sharing your activities and ideas!!" - Melissa C.
Shop eBooks of Activities

Get activity plans delivered to your inbox, every week!
Activities that hands-on parents absolutely love.

Top Physical Activities for Toddlers! Mom, Embrace the Energy!

6 Different Activities for 6 Lines of Tape

25 Sensory Activities for Kids with Sensory Tubs & Further Exploration

What Toddler Crafts & Art Projects Can We Do? 30 Ideas

How to Make a Lava Lamp Experiment Without Alka Seltzer

40+ Awesome Number Activities for Preschoolers
Get started having fun with your kids.
PLAN THE FUN WITH THE FREE KIDS ACTIVITIES PLANNER! AND RECEIVE ACTIVITIES EVERY WEEK!
- Preschoolers
- Kindergartners
- Grade School
- Literacy & ABCs
- Math & 123s
- Art Projects
- Gross Motor
- Shop Activity Plans
- Member Login
- Science, Tech, Math ›
- Chemistry ›
Lab Equipment & Instruments
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry Lab
This is a collection of lab equipment and scientific instruments.
Glassware Is Important for a Lab
Analytical balance.
This type of analytical balance is called a Mettler balance. This is a digital balance used for measuring mass with 0.1 mg precision.
Beakers in the Chemistry Lab
A centrifuge is a motorized piece of laboratory equipment which spins liquid samples to separate their components. Centrifuges come in two main sizes, a tabletop version which is often called a microcentrifuge and a larger floor model.
Laptop Computer
A computer is a valuable piece of modern laboratory equipment.
Flask Glassware Used for Medium Volumes
One characteristic that distinguishes flasks is that they present a narrow section called a neck.
Erlenmeyer Flasks
An Erlenmeyer flask is a type of laboratory flask with a conical base and cylindrical neck. The flask is named after its inventor, German chemist Emil Erlenmeyer , who made the first Erlenmeyer flask in 1861.
Florence Flask
A Florence flask or boiling flask is a round-bottom borosilicate glass container with thick walls, capable of withstanding temperature changes.
A fume hood or fume cupboard is a piece of laboratory equipment designed to limit exposure to dangerous fumes. The air inside the fume hood is either vented to the outside or else filtered and recirculated.
Microwave Oven
A microwave can be used to melt or heat many chemicals.
Paper Chromatography
Pipet or pipette for measuring small volumes.
Pipets (pipettes) are used to measure and transfer small volumes . There are many different types of pipets. Examples of pipet types include disposable, reusable, autoclavable, and manual
Graduated Cylinder
Thermometer, volumetric flask.
Volumetric flasks are used to accurately prepare solutions for chemistry.
Electronic Microscope
Funnel & flasks, micropipette, sample extraction.
A Petri dish is a shallow cylindrical dish that has a lid. It is named after its inventor, German bacteriologist Julius Petri. Petri dishes are made of glass or plastic.
Pipette Bulb
A pipette bulb is used to draw liquid up into a pipette.
Spectrophotometer
A spectrophotometer is a device capable of measuring light intensity as a function of its wavelength.
The titration also known as titrimetry or volumetric analysis is a process used for accurately measuring volume.
Example of a Chemistry Lab
Galileo thermometer.
A Galileo thermometer works using principles of buoyancy.
Bunsen Burner Picture
Chemostat bioreactor.
A chemostat is a type of bioreactor in which the chemical environment is held constant (static) by removing effluent while adding culture medium. Ideally, the volume of the system is unchanged.
Gold Leaf Electroscope Diagram
The gold leaf electroscope can detect static electricity. The charge on the metal cap passes into the stem and gold. The stem and the gold have the same electrical charge, so they repel each other, causing the gold foil to bend outward from the stem.
Photoelectric Effect Diagram
The photoelectric effect occurs when matter emits electrons upon absorbing electromagnetic radiation, such as light.
Gas Chromatograph Diagram
This is a generalized diagram of a gas chromatograph, an instrument used to separate the chemical components of a complex sample.
Bomb Calorimeter
A calorimeter is a device used to measure the heat change or heat capacity of chemical reactions or physical changes.
Goethe Barometer
A 'Goethe barometer' or storm glass, a type of water-based barometer. The sealed body of the glass barometer is filled with water, while the narrow spout is open to the atmosphere.
Weights or Masses
Spring weighing scale.
A spring weighing scale is used to determine the weight of an object from the displacement of the spring.
Steel Ruler
Thermometer with fahrenheit and celsius scales, desiccator and vacuum desiccator glassware.
A desiccator is sealed container which holds a desiccant to protect items or chemicals from humidity.
- Setting Up a Home Chemistry Lab
- What Is Chemical Engineering?
- How To Work With Glass Tubing in the Lab
- Check Out These Chemistry Career Options Before You Get a Degree
- How Is Carbon Fiber Made?
- All About Carbon Nanotubes
- Tips and Rules for Determining Significant Figures
- Useful Science Clipart and Diagrams
- How to Set Up Distillation Apparatus
- How To Design a Science Fair Experiment
- How to Use a Scientific Calculator
- Scientific Method Lesson Plan
- The Difference Between Control Group and Experimental Group
- What Is a Volumetric Flask?
- Scientific Hypothesis, Model, Theory, and Law
- Chemical Names of Common Substances

IMAGES
VIDEO
COMMENTS
The following is a list of historically important scientific experiments and observations demonstrating something of great scientific interest, typically in an elegant or clever manner. Astronomy. Ole Rømer makes the first quantitative estimate of the speed of light in 1676 by timing the motions of Jupiter's satellite Io with a telescope;
This is one easy science experiment that never fails to astonish. With carefully placed scissor cuts on an index card, you can make a loop large enough to fit a (small) human body through! Kids will be wowed as they learn about surface area. Science Sparks. 68. Stand on a pile of paper cups.
"Double-slit experiments have become so compelling [because] they are relatively easy to conduct," says David Kaiser, a professor of physics and of the history of science at MIT. "There is an unusually large ratio, in this case, between the relative simplicity and accessibility of the experimental design and the deep conceptual ...
Fun science experiments to explore everything from kitchen chemistry to DIY mini drones. Easy to set up and perfect for home or school. Browse the collection and see what you want to try first!
1020 - Avicenna (Ibn Sina) introduces experimentation and quantification into the study of medicine and physiology, including the introduction of experimental medicine and clinical trials, in The Canon of Medicine. 1021 - Ibn al-Haytham (Alhacen) pioneers the experimental scientific method and experimental physics in his Book of Optics, where he devises the first scientific experiments on ...
But, as science has advanced, there is clear evidence to argue against that. The answer to this question is complicated because evolution is both fact and theory. According to the National Center for Science Education, scientific understanding needs both theories and facts. There is proof that organisms have changed or evolved over time, and ...
This experiment provides a firsthand experience of how the density and composition of gases can influence sound transmission. It encourages scientific curiosity, observation, and a sense of wonder as students witness the surprising transformation of their voices. 58. Liquid Nitrogen Ice Cream
Science experiments you can do at home! Explore an ever growing list of hundreds of fun and easy science experiments. Have fun trying these experiments at home or use them for science fair project ideas. Explore experiments by category, newest experiments, most popular experiments, easy at home experiments, or simply scroll down this page for tons of awesome experiment ideas!
The six steps of the scientific method include: 1) asking a question about something you observe, 2) doing background research to learn what is already known about the topic, 3) constructing a hypothesis, 4) experimenting to test the hypothesis, 5) analyzing the data from the experiment and drawing conclusions, and 6) communicating the results ...
The atom-splitting experiments included J.J. Thomson's discovery of the electron in 1897, Ernest Rutherford and Frederick Soddy's "transmutation" experiments (converting atoms into other atoms), James Chadwick's 1932 discovery of the neutron, and one of the most famous experiments of them all: the 1909 Geiger-Marsden or gold-foil experiment.
Turn this into a 7th grade science fair project by changing up the variables (does temperature matter?) or items being electroplated. Learn more: Electroplating at KiwiCo. Swab and test for germs Angelic Scalliwags. Difficulty: Medium / Materials: Medium. Germ experiments are one of the most popular science fair ideas for 7th grade students.
The Top Ten Scientific Discoveries of the Decade. Breakthroughs include measuring the true nature of the universe, finding new species of human ancestors, and unlocking new ways to fight disease.
32. DIY Science Experiment. The best science experiment your child can engage in is the one they create themselves! Begin brainstorming a list of questions and let the world be their oyster as they plan and carry out their own experiments. Some of our favorite brainstorming questions, from Scholastic's Science-Fair Project Guide, are listed ...
This one will really get them into brushing their teeth once they scientifically prove all the good things that toothpaste can do. Write on sticky notes: Soda 1, Soda 2, Juice 1, and Juice 2.
A laboratory is a special room or place that is equipped to facilitate scientific experiments, observations and for teaching science. Laboratory apparatus refers to the various tools, equipment, and instruments used in scientific research, experimentation, and analysis within a laboratory setting. These tools are essential for conducting experiments, measuring and analyzing data, and ensuring ...
Both laws and theories depend on basic elements of the scientific method, such as generating a hypothesis, testing that premise, finding (or not finding) empirical evidence and coming up with conclusions.Eventually, other scientists must be able to replicate the results if the experiment is destined to become the basis for a widely accepted law or theory.
Dive into 100 easy science experiments for kids to do at home, featuring activities like Traveling Rainbows, making slime, exploring colors with baking soda and vinegar, and revealing secret messages with invisible ink. Perfect for curious minds eager to learn through fun, hands-on science.
Here are the best winter science experiments perfect for kids of all ages.. When spring is around the corner, try these spring science experiments!. During the summer, don't let science learning slip! Instead, do these summer science experiments.. And this fall, try some of the fun fall science experiments on this list.. Science Experiments by Grade
Fun science experiments to explore everything from kitchen chemistry to DIY mini drones. Easy to set up and perfect for home or school. Browse the collection and see what you want to try first!
Plant Themed Simple Science Experiments. Enjoy learning about seeds, plant parts, and how plants grow with these simple science experiments. Learn about how plants soak up water through their stems with a flower experiment for kids from Growing A Jeweled Rose.; Watch seeds sprout as you grow seeds in a jar as seen on Teaching Mama.; Learn about the parts of the seed with a seed coat experiment ...
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. ... How To Design a Science Fair Experiment. How to Use a Scientific Calculator. Scientific Method Lesson Plan. The Difference Between Control Group and ...