- Research article
- Open access
- Published: 16 November 2020

Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews
- Pawel Posadzki 1 , 2 ,
- Dawid Pieper ORCID: orcid.org/0000-0002-0715-5182 3 ,
- Ram Bajpai 4 ,
- Hubert Makaruk 5 ,
- Nadja Könsgen 3 ,
- Annika Lena Neuhaus 3 &
- Monika Semwal 6
BMC Public Health volume 20 , Article number: 1724 ( 2020 ) Cite this article
36k Accesses
150 Citations
147 Altmetric
Metrics details
Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity for various health outcomes.
Overview and meta-analysis. The Cochrane Library was searched from 01.01.2000 to issue 1, 2019. No language restrictions were imposed. Only CSRs of randomised controlled trials (RCTs) were included. Both healthy individuals, those at risk of a disease, and medically compromised patients of any age and gender were eligible. We evaluated any type of exercise or physical activity interventions; against any types of controls; and measuring any type of health-related outcome measures. The AMSTAR-2 tool for assessing the methodological quality of the included studies was utilised.
Hundred and fifty CSRs met the inclusion criteria. There were 54 different conditions. Majority of CSRs were of high methodological quality. Hundred and thirty CSRs employed meta-analytic techniques and 20 did not. Limitations for studies were the most common reasons for downgrading the quality of the evidence. Based on 10 CSRs and 187 RCTs with 27,671 participants, there was a 13% reduction in mortality rates risk ratio (RR) 0.87 [95% confidence intervals (CI) 0.78 to 0.96]; I 2 = 26.6%, [prediction interval (PI) 0.70, 1.07], median effect size (MES) = 0.93 [interquartile range (IQR) 0.81, 1.00]. Data from 15 CSRs and 408 RCTs with 32,984 participants showed a small improvement in quality of life (QOL) standardised mean difference (SMD) 0.18 [95% CI 0.08, 0.28]; I 2 = 74.3%; PI -0.18, 0.53], MES = 0.20 [IQR 0.07, 0.39]. Subgroup analyses by the type of condition showed that the magnitude of effect size was the largest among patients with mental health conditions.
There is a plethora of CSRs evaluating the effectiveness of physical activity/exercise. The evidence suggests that physical activity/exercise reduces mortality rates and improves QOL with minimal or no safety concerns.
Trial registration
Registered in PROSPERO ( CRD42019120295 ) on 10th January 2019.
Peer Review reports
The World Health Organization (WHO) defines physical activity “as any bodily movement produced by skeletal muscles that requires energy expenditure” [ 1 ]. Therefore, physical activity is not only limited to sports but also includes walking, running, swimming, gymnastics, dance, ball games, and martial arts, for example. In the last years, several organizations have published or updated their guidelines on physical activity. For example, the Physical Activity Guidelines for Americans, 2nd edition, provides information and guidance on the types and amounts of physical activity that provide substantial health benefits [ 2 ]. The evidence about the health benefits of regular physical activity is well established and so are the risks of sedentary behaviour [ 2 ]. Exercise is dose dependent, meaning that people who achieve cumulative levels several times higher than the current recommended minimum level have a significant reduction in the risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events [ 3 ]. Benefits of physical activity have been reported for numerous outcomes such as mortality [ 4 , 5 ], cognitive and physical decline [ 5 , 6 , 7 ], glycaemic control [ 8 , 9 ], pain and disability [ 10 , 11 ], muscle and bone strength [ 12 ], depressive symptoms [ 13 ], and functional mobility and well-being [ 14 , 15 ]. Overall benefits of exercise apply to all bodily systems including immunological [ 16 ], musculoskeletal [ 17 ], respiratory [ 18 ], and hormonal [ 19 ]. Specifically for the cardiovascular system, exercise increases fatty acid oxidation, cardiac output, vascular smooth muscle relaxation, endothelial nitric oxide synthase expression and nitric oxide availability, improves plasma lipid profiles [ 15 ] while at the same time reducing resting heart rate and blood pressure, aortic valve calcification, and vascular resistance [ 20 ].
However, the degree of all the above-highlighted benefits vary considerably depending on individual fitness levels, types of populations, age groups and the intensity of different physical activities/exercises [ 21 ]. The majority of guidelines in different countries recommend a goal of 150 min/week of moderate-intensity aerobic physical activity (or equivalent of 75 min of vigorous-intensity) [ 22 ] with differences for cardiovascular disease [ 23 ] or obesity prevention [ 24 ] or age groups [ 25 ].
There is a plethora of systematic reviews published by the Cochrane Library critically evaluating the effectiveness of physical activity/exercise for various health outcomes. Cochrane systematic reviews (CSRs) are known to be a source of high-quality evidence. Thus, it is not only timely but relevant to evaluate the current knowledge, and determine the quality of the evidence-base, and the magnitude of the effect sizes given the negative lifestyle changes and rising physical inactivity-related burden of diseases. This overview will identify the breadth and scope to which CSRs have appraised the evidence for exercise on health outcomes; and this will help in directing future guidelines and identifying current gaps in the literature.
The objectives of this research were to a. answer the following research questions: in children, adolescents and adults (both healthy and medically compromised) what are the effects (and adverse effects) of exercise/physical activity in improving various health outcomes (e.g., pain, function, quality of life) reported in CSRs; b. estimate the magnitude of the effects by pooling the results quantitatively; c. evaluate the strength and quality of the existing evidence; and d. create recommendations for future researchers, patients, and clinicians.
Our overview was registered with PROSPERO (CRD42019120295) on 10th January 2019. The Cochrane Handbook for Systematic Reviews of interventions and Preferred Reporting Items for Overviews of Reviews were adhered to while writing and reporting this overview [ 26 , 27 ].
Search strategy and selection criteria
We followed the practical guidance for conducting overviews of reviews of health care interventions [ 28 ] and searched the Cochrane Database of Systematic Reviews (CDSR), 2019, Issue 1, on the Cochrane Library for relevant papers using the search strategy: (health) and (exercise or activity or physical). The decision to seek CSRs only was based on three main aspects. First, high quality (CSRs are considered to be the ‘gold methodological standard’) [ 29 , 30 , 31 ]. Second, data saturation (enough high-quality evidence to reach meaningful conclusions based on CSRs only). Third, including non-CSRs would have heavily increased the issue of overlapping reviews (also affecting data robustness and credibility of conclusions). One reviewer carried out the searches. The study screening and selection process were performed independently by two reviewers. We imported all identified references into reference manager software EndNote (X8). Any disagreements were resolved by discussion between the authors with third overview author acting as an arbiter, if necessary.
We included CSRs of randomised controlled trials (RCTs) involving both healthy individuals and medically compromised patients of any age and gender. Only CSRs assessing exercise or physical activity as a stand-alone intervention were included. This included interventions that could initially be taught by a professional or involve ongoing supervision (the WHO definition). Complex interventions e.g., assessing both exercise/physical activity and behavioural changes were excluded if the health effects of the interventions could not have been attributed to exercise distinctly.
Any types of controls were admissible. Reviews evaluating any type of health-related outcome measures were deemed eligible. However, we excluded protocols or/and CSRs that have been withdrawn from the Cochrane Library as well as reviews with no included studies.
Data analysis
Three authors (HM, ALN, NK) independently extracted relevant information from all the included studies using a custom-made data collection form. The methodological quality of SRs included was independently evaluated by same reviewers using the AMSTAR-2 tool [ 32 ]. Any disagreements on data extraction or CSR quality were resolved by discussion. The entire dataset was validated by three authors (PP, MS, DP) and any discrepant opinions were settled through discussions.
The results of CSRs are presented in a narrative fashion using descriptive tables. Where feasible, we presented outcome measures across CSRs. Data from the subset of homogeneous outcomes were pooled quantitatively using the approach previously described by Bellou et al. and Posadzki et al. [ 33 , 34 ]. For mortality and quality of life (QOL) outcomes, the number of participants and RCTs involved in the meta-analysis, summary effect sizes [with 95% confidence intervals (CI)] using random-effects model were calculated. For binary outcomes, we considered relative risks (RRs) as surrogate measures of the corresponding odds ratio (OR) or risk ratio/hazard ratio (HR). To stabilise the variance and normalise the distributions, we transformed RRs into their natural logarithms before pooling the data (a variation was allowed, however, it did not change interpretation of results) [ 35 ]. The standard error (SE) of the natural logarithm of RR was derived from the corresponding CIs, which was either provided in the study or calculated with standard formulas [ 36 ]. Binary outcomes reported as risk difference (RD) were also meta-analysed if two more estimates were available. For continuous outcomes, we only meta-analysed estimates that were available as standardised mean difference (SMD), and estimates reported with mean differences (MD) for QOL were presented separately in a supplementary Table 9 . To estimate the overall effect size, each study was weighted by the reciprocal of its variance. Random-effects meta-analysis, using DerSimonian and Laird method [ 37 ] was applied to individual CSR estimates to obtain a pooled summary estimate for RR or SMD. The 95% prediction interval (PI) was also calculated (where ≥3 studies were available), which further accounts for between-study heterogeneity and estimates the uncertainty around the effect that would be anticipated in a new study evaluating that same association. I -squared statistic was used to measure between study heterogeneity; and its various thresholds (small, substantial and considerable) were interpreted considering the size and direction of effects and the p -value from Cochran’s Q test ( p < 0.1 considered as significance) [ 38 ]. Wherever possible, we calculated the median effect size (with interquartile range [IQR]) of each CSR to interpret the direction and magnitude of the effect size. Sub-group analyses are planned for type and intensity of the intervention; age group; gender; type and/or severity of the condition, risk of bias in RCTs, and the overall quality of the evidence (Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria). To assess overlap we calculated the corrected covered area (CCA) [ 39 ]. All statistical analyses were conducted on Stata statistical software version 15.2 (StataCorp LLC, College Station, Texas, USA).
The searches generated 280 potentially relevant CRSs. After removing of duplicates and screening, a total of 150 CSRs met our eligibility criteria [ 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 ] (Fig. 1 ). Reviews were published between September 2002 and December 2018. A total of 130 CSRs employed meta-analytic techniques and 20 did not. The total number of RCTs in the CSRs amounted to 2888; with 485,110 participants (mean = 3234, SD = 13,272). The age ranged from 3 to 87 and gender distribution was inestimable. The main characteristics of included reviews are summarised in supplementary Table 1 . Supplementary Table 2 summarises the effects of physical activity/exercise on health outcomes. Conclusions from CSRs are listed in supplementary Table 3 . Adverse effects are listed in supplementary Table 4 . Supplementary Table 5 presents summary of withdrawals/non-adherence. The methodological quality of CSRs is presented in supplementary Table 6 . Supplementary Table 7 summarises studies assessed at low risk of bias (by the authors of CSRs). GRADE-ings of the review’s main comparison are listed in supplementary Table 8 .
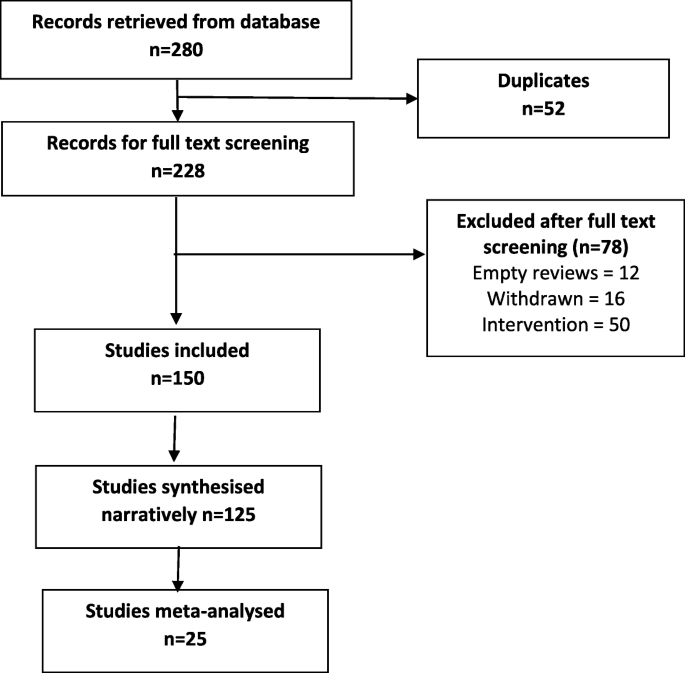
Study selection process
There were 54 separate populations/conditions, considerable range of interventions and comparators, co-interventions, and outcome measures. For detailed description of interventions, please refer to the supplementary tables . Most commonly measured outcomes were - function 112 (75%), QOL 83 (55%), AEs 70 (47%), pain 41 (27%), mortality 28 (19%), strength 30 (20%), costs 47 (31%), disability 14 (9%), and mental health in 35 (23%) CSRs.
There was a 13% reduction in mortality rates risk ratio (RR) 0.87 [95% CI 0.78 to 0.96]; I 2 = 26.6%, [PI 0.70, 1.07], median effect size (MES) = 0.93 [interquartile range (IQR) 0.81, 1.00]; 10 CSRs, 187 RCTs, 27,671 participants) following exercise when compared with various controls (Table 1 ). This reduction was smaller in ‘other groups’ of patients when compared to cardiovascular diseases (CVD) patients - RR 0.97 [95% CI 0.65, 1.45] versus 0.85 [0.76, 0.96] respectively. The effects of exercise were not intensity or frequency dependent. Sessions more than 3 times per week exerted a smaller reduction in mortality as compared with sessions of less than 3 times per week RR 0.87 [95% CI 0.78, 0.98] versus 0.63 [0.39, 1.00]. Subgroup analyses by risk of bias (ROB) in RCTs showed that RCTs at low ROB exerted smaller reductions in mortality when compared to RCTs at an unclear or high ROB, RR 0.90 [95% CI 0.78, 1.02] versus 0.72 [0.42, 1.22] versus 0.86 [0.69, 1.06] respectively. CSRs with moderate quality of evidence (GRADE), showed slightly smaller reductions in mortality when compared with CSRs that relied on very low to low quality evidence RR 0.88 [95% CI 0.79, 0.98] versus 0.70 [0.47, 1.04].
Exercise also showed an improvement in QOL, standardised mean difference (SMD) 0.18 [95% CI 0.08, 0.28]; I 2 = 74.3%; PI -0.18, 0.53], MES = 0.20 [IQR 0.07, 0.39]; 15 CSRs, 408 RCTs, 32,984 participants) when compared with various controls (Table 2 ). These improvements were greater observed for health related QOL when compared to overall QOL SMD 0.30 [95% CI 0.21, 0.39] vs 0.06 [− 0.08, 0.20] respectively. Again, the effects of exercise were duration and frequency dependent. For instance, sessions of more than 90 mins exerted a greater improvement in QOL as compared with sessions up to 90 min SMD 0.24 [95% CI 0.11, 0.37] versus 0.22 [− 0.30, 0.74]. Subgroup analyses by the type of condition showed that the magnitude of effect was the largest among patients with mental health conditions, followed by CVD and cancer. Physical activity exerted negative effects on QOL in patients with respiratory conditions (2 CSRs, 20 RCTs with 601 patients; SMD -0.97 [95% CI -1.43, 0.57]; I 2 = 87.8%; MES = -0.46 [IQR-0.97, 0.05]). Subgroup analyses by risk of bias (ROB) in RCTs showed that RCTs at low or unclear ROB exerted greater improvements in QOL when compared to RCTs at a high ROB SMD 0.21 [95% CI 0.10, 0.31] versus 0.17 [0.03, 0.31]. Analogically, CSRs with moderate to high quality of evidence showed slightly greater improvements in QOL when compared with CSRs that relied on very low to low quality evidence SMD 0.19 [95% CI 0.05, 0.33] versus 0.15 [− 0.02, 0.32]. Please also see supplementary Table 9 more studies reporting QOL outcomes as mean difference (not quantitatively synthesised herein).
Adverse events (AEs) were reported in 100 (66.6%) CSRs; and not reported in 50 (33.3%). The number of AEs ranged from 0 to 84 in the CSRs. The number was inestimable in 83 (55.3%) CSRs. Ten (6.6%) reported no occurrence of AEs. Mild AEs were reported in 28 (18.6%) CSRs, moderate in 9 (6%) and serious/severe in 20 (13.3%). There were 10 deaths and in majority of instances, the causality was not attributed to exercise. For this outcome, we were unable to pool the data as effect sizes were too heterogeneous (Table 3 ).
In 38 CSRs, the total number of trials reporting withdrawals/non-adherence was inestimable. There were different ways of reporting it such as adherence or attrition (high in 23.3% of CSRs) as well as various effect estimates including %, range, total numbers, MD, RD, RR, OR, mean and SD. The overall pooled estimates are reported in Table 3 .
Of all 16 domains of the AMSTAR-2 tool, 1876 (78.1%) scored ‘yes’, 76 (3.1%) ‘partial yes’; 375 (15.6%) ‘no’, and ‘not applicable’ in 25 (1%) CSRs. Ninety-six CSRs (64%) were scored as ‘no’ on reporting sources of funding for the studies followed by 88 (58.6%) failing to explain the selection of study designs for inclusion. One CSR (0.6%) each were judged as ‘no’ for reporting any potential sources of conflict of interest, including any funding for conducting the review as well for performing study selection in duplicate.
In 102 (68%) CSRs, there was predominantly a high risk of bias in RCTs. In 9 (6%) studies, this was reported as a range, e.g., low or unclear or low to high. Two CSRs used different terminology i.e., moderate methodological quality; and the risk of bias was inestimable in one CSR. Sixteen (10.6%) CSRs did not identify any studies (RCTs) at low risk of random sequence generation, 28 (18.6%) allocation concealment, 28 (18.6%) performance bias, 84 (54%) detection bias, 35 (23.3%) attrition bias, 18 (12%) reporting bias, and 29 (19.3%) other bias.
In 114 (76%) CSRs, limitation of studies was the main reason for downgrading the quality of the evidence followed by imprecision in 98 (65.3%) and inconsistency in 68 (45.3%). Publication bias was the least frequent reason for downgrading in 26 (17.3%) CSRs. Ninety-one (60.7%) CSRs reached equivocal conclusions, 49 (32.7%) reviews reached positive conclusions and 10 (6.7%) reached negative conclusions (as judged by the authors of CSRs).
In this systematic review of CSRs, we found a large body of evidence on the beneficial effects of physical activity/exercise on health outcomes in a wide range of heterogeneous populations. Our data shows a 13% reduction in mortality rates among 27,671 participants, and a small improvement in QOL and health-related QOL following various modes of physical activity/exercises. This means that both healthy individuals and medically compromised patients can significantly improve function, physical and mental health; or reduce pain and disability by exercising more [ 190 ]. In line with previous findings [ 191 , 192 , 193 , 194 ], where a dose-specific reduction in mortality has been found, our data shows a greater reduction in mortality in studies with longer follow-up (> 12 months) as compared to those with shorter follow-up (< 12 months). Interestingly, we found a consistent pattern in the findings, the higher the quality of evidence and the lower the risk of bias in primary studies, the smaller reductions in mortality. This pattern is observational in nature and cannot be over-generalised; however this might mean less certainty in the estimates measured. Furthermore, we found that the magnitude of the effect size was the largest among patients with mental health conditions. A possible mechanism of action may involve elevated levels of brain-derived neurotrophic factor or beta-endorphins [ 195 ].
We found the issue of poor reporting or underreporting of adherence/withdrawals in over a quarter of CSRs (25.3%). This is crucial both for improving the accuracy of the estimates at the RCT level as well as maintaining high levels of physical activity and associated health benefits at the population level.
Even the most promising interventions are not entirely risk-free; and some minor AEs such as post-exercise pain and soreness or discomfort related to physical activity/exercise have been reported. These were typically transient; resolved within a few days; and comparable between exercise and various control groups. However worryingly, the issue of poor reporting or underreporting of AEs has been observed in one third of the CSRs. Transparent reporting of AEs is crucial for identifying patients at risk and mitigating any potential negative or unintended consequences of the interventions.
High risk of bias of the RCTs evaluated was evident in more than two thirds of the CSRs. For example, more than half of reviews identified high risk of detection bias as a major source of bias suggesting that lack of blinding is still an issue in trials of behavioural interventions. Other shortcomings included insufficiently described randomisation and allocation concealment methods and often poor outcome reporting. This highlights the methodological challenges in RCTs of exercise and the need to counterbalance those with the underlying aim of strengthening internal and external validity of these trials.
Overall, high risk of bias in the primary trials was the main reason for downgrading the quality of the evidence using the GRADE criteria. Imprecision was frequently an issue, meaning the effective sample size was often small; studies were underpowered to detect the between-group differences. Pooling too heterogeneous results often resulted in inconsistent findings and inability to draw any meaningful conclusions. Indirectness and publication bias were lesser common reasons for downgrading. However, with regards to the latter, the generally accepted minimum number of 10 studies needed for quantitatively estimate the funnel plot asymmetry was not present in 69 (46%) CSRs.
Strengths of this research are the inclusion of large number of ‘gold standard’ systematic reviews, robust screening, data extractions and critical methodological appraisal. Nevertheless, some weaknesses need to be highlighted when interpreting findings of this overview. For instance, some of these CSRs analysed the same primary studies (RCTs) but, arrived at slightly different conclusions. Using, the Pieper et al. [ 39 ] formula, the amount of overlap ranged from 0.01% for AEs to 0.2% for adherence, which indicates slight overlap. All CSRs are vulnerable to publication bias [ 196 ] - hence the conclusions generated by them may be false-positive. Also, exercise was sometimes part of a complex intervention; and the effects of physical activity could not be distinguished from co-interventions. Often there were confounding effects of diet, educational, behavioural or lifestyle interventions; selection, and measurement bias were inevitably inherited in this overview too. Also, including CSRs only might lead to selection bias; and excluding reviews published before 2000 might limit the overall completeness and applicability of the evidence. A future update should consider these limitations, and in particular also including non-CSRs.
Conclusions
Trialists must improve the quality of primary studies. At the same time, strict compliance with the reporting standards should be enforced. Authors of CSRs should better explain eligibility criteria and report sources of funding for the primary studies. There are still insufficient physical activity trends worldwide amongst all age groups; and scalable interventions aimed at increasing physical activity levels should be prioritized [ 197 ]. Hence, policymakers and practitioners need to design and implement comprehensive and coordinated strategies aimed at targeting physical activity programs/interventions, health promotion and disease prevention campaigns at local, regional, national, and international levels [ 198 ].
Availability of data and materials
Data sharing is not applicable to this article as no raw data were analysed during the current study. All information in this article is based on published systematic reviews.
Abbreviations
Adverse events
Cardiovascular diseases
Cochrane Database of Systematic Reviews
Cochrane systematic reviews
Confidence interval
Grading of Recommendations Assessment, Development and Evaluation
Hazard ratio
Interquartile range
Mean difference
Prediction interval
Quality of life
Randomised controlled trials
Relative risk
Risk difference
Risk of bias
Standard error
Standardised mean difference
World Health Organization
https://www.who.int/dietphysicalactivity/pa/en/ . (Accessed 8 June 2020).
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for AmericansPhysical activity guidelines for AmericansPhysical activity guidelines for Americans. Jama. 2018;320(19):2020–8.
PubMed Google Scholar
Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. BMJ. 2016;354:i3857.
PubMed PubMed Central Google Scholar
Abell B, Glasziou P, Hoffmann T. The contribution of individual exercise training components to clinical outcomes in randomised controlled trials of cardiac rehabilitation: a systematic review and meta-regression. Sports Med Open. 2017;3(1):19.
Anderson D, Seib C, Rasmussen L. Can physical activity prevent physical and cognitive decline in postmenopausal women? A systematic review of the literature. Maturitas. 2014;79(1):14–33.
Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic review. Physiother Can. 2010;62(1):25–34.
Barlow PA, Otahal P, Schultz MG, Shing CM, Sharman JE. Low exercise blood pressure and risk of cardiovascular events and all-cause mortality: systematic review and meta-analysis. Atherosclerosis. 2014;237(1):13–22.
CAS PubMed Google Scholar
Aljawarneh YM, Wardell DW, Wood GL, Rozmus CL. A systematic review of physical activity and exercise on physiological and biochemical outcomes in children and adolescents with type 1 diabetes. J Nurs Scholarsh. 2019.
Chastin SFM, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M, Stamatakis E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370–6.
Abdulla SY, Southerst D, Cote P, Shearer HM, Sutton D, Randhawa K, Varatharajan S, Wong JJ, Yu H, Marchand AA, et al. Is exercise effective for the management of subacromial impingement syndrome and other soft tissue injuries of the shoulder? A systematic review by the Ontario protocol for traffic injury management (OPTIMa) collaboration. Man Ther. 2015;20(5):646–56.
Alanazi MH, Parent EC, Dennett E. Effect of stabilization exercise on back pain, disability and quality of life in adults with scoliosis: a systematic review. Eur J Phys Rehabil Med. 2018;54(5):647–53.
Adsett JA, Mudge AM, Morris N, Kuys S, Paratz JD. Aquatic exercise training and stable heart failure: a systematic review and meta-analysis. Int J Cardiol. 2015;186:22–8.
Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(7):1329–38.
Abdin S, Welch RK, Byron-Daniel J, Meyrick J. The effectiveness of physical activity interventions in improving well-being across office-based workplace settings: a systematic review. Public Health. 2018;160:70–6.
Albalawi H, Coulter E, Ghouri N, Paul L. The effectiveness of structured exercise in the south Asian population with type 2 diabetes: a systematic review. Phys Sportsmed. 2017;45(4):408–17.
Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, Bragazzi N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187.
Hagen KB, Dagfinrud H, Moe RH, Østerås N, Kjeken I, Grotle M, Smedslund G. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Med. 2012;10(1):167.
Burton DA, Stokes K, Hall GM. Physiological effects of exercise. Contin Educ Anaesth Crit Care Pain. 2004;4(6):185–8.
Google Scholar
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–61.
Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135.
CAS PubMed PubMed Central Google Scholar
Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12.
Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–56.
Excellence NIfHaC: Cardiovascular disease prevention public health guideline [PH25] Published date: June 2010. Available at: https://www.nice.org.uk/guidance/ph25/resources/cardiovascular-disease-prevention-pdf-1996238687173 .
Excellence NIfHaC: Obesity prevention clinical guideline [CG43] published date: December 2006 Last updated: March 2015. Available at: https://www.nice.org.uk/guidance/cg43/resources/obesity-prevention-pdf-975445344709 .
Excellence NIfHaC: Physical activity for children and young people public health guideline [PH17] Published date: January 2009. Available at: https://www.nice.org.uk/guidance/ph17/resources/physical-activity-for-children-and-young-people-pdf-1996181580229 .
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 6 [updated September 2018] edition. Available from www.cochrane-handbook.org : The Cochrane Collaboration, 2011. 2011.
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich A-B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24.
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18.
Handoll H, Madhok R. Quality of Cochrane reviews. Another study found that most Cochrane reviews are of a good standard. BMJ. 2002;324(7336):545.
Petticrew M, Wilson P, Wright K, Song F. Quality of Cochrane reviews. Quality of Cochrane reviews is better than that of non-Cochrane reviews. BMJ. 2002;324(7336):545.
Shea B, Moher D, Graham I, Pham B, Tugwell P. A comparison of the quality of Cochrane reviews and systematic reviews published in paper-based journals. Eval Health Prof. 2002;25(1):116–29.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Posadzki PP, Bajpai R, Kyaw BM, Roberts NJ, Brzezinski A, Christopoulos GI, Divakar U, Bajpai S, Soljak M, Dunleavy G, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018;16(1):18.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9.
Walter SD, Cook RJ. A comparison of several point estimators of the odds ratio in a single 2 x 2 contingency table. Biometrics. 1991;47(3):795–811.
Khan H, Sempos CT. Statistical methods in epidemiology. New York: Oxford University Press; 1989.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
CAS Google Scholar
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019): Cochrane; 2019. Available from www.training.cochrane.org/handbook .
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;7.
Al-Khudairy L, Loveman E, Colquitt JL, Mead E, Johnson RE, Fraser H, Olajide J, Murphy M, Velho RM, O'Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017;6.
Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013;7.
Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4.
Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;1.
Andriolo RB, El Dib RP, Ramos L, Atallah Á, da Silva EMK. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database Syst Rev. 2010;5.
Araujo DN, Ribeiro CTD, Maciel ACC, Bruno SS, Fregonezi GAF, Dias FAL. Physical exercise for the treatment of non-ulcerated chronic venous insufficiency. Cochrane Database Syst Rev. 2016;12.
Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;1.
Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsøe B, Dagfinrud H, Lund H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3.
Beggs S, Foong YC, Le HCT, Noor D, Wood-Baker R, Walters JAE. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev. 2013;4.
Bergenthal N, Will A, Streckmann F, Wolkewitz KD, Monsef I, Engert A, Elter T, Skoetz N. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014;11.
Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Góes SM, Boden C, Foulds HJA. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;6.
Bidonde J, Busch AJ, van der Spuy I, Tupper S, Kim SY, Boden C. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst Rev. 2017;9.
Bidonde J, Busch AJ, Webber SC, Schachter CL, Danyliw A, Overend TJ, Richards RS, Rader T. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;10.
Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3.
Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2015;1.
Broderick J, Crumlish N, Waugh A, Vancampfort D. Yoga versus non-standard care for schizophrenia. Cochrane Database Syst Rev. 2017;9.
Broderick J, Knowles A, Chadwick J, Vancampfort D. Yoga versus standard care for schizophrenia. Cochrane Database Syst Rev. 2015;10.
Broderick J, Vancampfort D. Yoga as part of a package of care versus standard care for schizophrenia. Cochrane Database Syst Rev. 2017;9.
Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. 2017;6.
Busch AJ, Barber KA, Overend TJ, Peloso PMJ, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;4.
Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, Danyliw A, Sawant A, Dal Bello-Haas V, Rader T, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;12.
Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9.
Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;9.
Carvalho APV, Vital FMR, Soares BGO. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev. 2012;4.
Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6.
Cavalheri V, Tahirah F, Nonoyama ML, Jenkins S, Hill K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev. 2013;7.
Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database Syst Rev. 2006;3.
Choi BKL, Verbeek JH, Tam WWS, Jiang JY. Exercises for prevention of recurrences of low-back pain. Cochrane Database Syst Rev. 2010;1.
Colquitt JL, Loveman E, O'Malley C, Azevedo LB, Mead E, Al-Khudairy L, Ells LJ, Metzendorf MI, Rees K. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database Syst Rev. 2016;3.
Connolly B, Salisbury L, O'Neill B, Geneen LJ, Douiri A, Grocott MPW, Hart N, Walsh TS, Blackwood B. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev. 2015;6.
Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE. Exercise for depression. Cochrane Database Syst Rev. 2013;9.
Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev. 2015;10.
Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1.
Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11.
Dal Bello-Haas V, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev. 2013;5.
Dale MT, McKeough ZJ, Troosters T, Bye P, Alison JA. Exercise training to improve exercise capacity and quality of life in people with non-malignant dust-related respiratory diseases. Cochrane Database Syst Rev. 2015;11.
Daley A, Stokes-Lampard H, Thomas A, MacArthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;11.
de Morton N, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;1.
Dobbins M, Husson H, DeCorby K, LaRocca RL. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database Syst Rev. 2013;2.
Doiron KA, Hoffmann TC, Beller EM. Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev. 2018;3.
Ekeland E, Heian F, Hagen KB, Abbott JM, Nordheim L. Exercise to improve self-esteem in children and young people. Cochrane Database Syst Rev. 2004;1.
Elbers RG, Verhoef J, van Wegen EEH, Berendse HW, Kwakkel G. Interventions for fatigue in Parkinson's disease. Cochrane Database Syst Rev. 2015;10.
Felbel S, Meerpohl JJ, Monsef I, Engert A, Skoetz N. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev. 2014;6.
Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4.
Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1.
Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4.
Freitas DA, Holloway EA, Bruno SS, Chaves GSS, Fregonezi GAF, Mendonça K. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2013;10.
Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9.
Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev. 2013;1.
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9.
Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Database Syst Rev. 2010;5.
Grande AJ, Keogh J, Hoffmann TC, Beller EM, Del Mar CB. Exercise versus no exercise for the occurrence, severity and duration of acute respiratory infections. Cochrane Database Syst Rev. 2015;6.
Grande AJ, Reid H, Thomas EE, Nunan D, Foster C. Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults. Cochrane Database Syst Rev. 2016;8.
Grande AJ, Silva V, Andriolo BNG, Riera R, Parra SA, Peccin MS. Water-based exercise for adults with asthma. Cochrane Database Syst Rev. 2014;7.
Gross A, Kay TM, Paquin JP, Blanchette S, Lalonde P, Christie T, Dupont G, Graham N, Burnie SJ, Gelley G, et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev. 2015;1.
Hageman D, Fokkenrood HJP, Gommans LNM, van den Houten MML, Teijink JAW. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4.
Han A, Judd M, Welch V, Wu T, Tugwell P, Wells GA. Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2004;3.
Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7.
Hartley L, Dyakova M, Holmes J, Clarke A, Lee MS, Ernst E, Rees K. Yoga for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;5.
Hartley L, Flowers N, Lee MS, Ernst E, Rees K. Tai chi for primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;4.
Hartley L, Lee MS, Kwong JSW, Flowers N, Todkill D, Ernst E, Rees K. Qigong for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;6.
Hassett L, Moseley AM, Harmer AR. Fitness training for cardiorespiratory conditioning after traumatic brain injury. Cochrane Database Syst Rev. 2017;12.
Hayden J, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;3.
Hay-Smith EJC, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2011;12.
Heine M, van de Port I, Rietberg MB, van Wegen EEH, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015;9.
Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;10.
Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué i Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12.
Herbert RD, de Noronha M, Kamper SJ. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst Rev. 2011;7.
Heymans MW, van Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for non-specific low-back pain. Cochrane Database Syst Rev. 2004;4.
Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;10.
Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7.
Hurkmans E, van der Giesen FJ, Vliet Vlieland TPM, Schoones J, Van den Ende E. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. 2009;4.
Hurley M, Dickson K, Hallett R, Grant R, Hauari H, Walsh N, Stansfield C, Oliver S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4.
Jones M, Harvey A, Marston L, O'Connell NE. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013;5.
Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev. 2015;10.
Kendrick D, Kumar A, Carpenter H, Zijlstra GAR, Skelton DA, Cook JR, Stevens Z, Belcher CM, Haworth D, Gawler SJ, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;11.
Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;3.
Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1.
Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12.
Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2017;4.
Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3.
Lauret GJ, Fakhry F, Fokkenrood HJP, Hunink MGM, Teijink JAW, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev. 2014;7.
Lawrence M, Celestino Junior FT, Matozinho HHS, Govan L, Booth J, Beecher J. Yoga for stroke rehabilitation. Cochrane Database Syst Rev. 2017;12.
Lin CWC, Donkers NAJ, Refshauge KM, Beckenkamp PR, Khera K, Moseley AM. Rehabilitation for ankle fractures in adults. Cochrane Database Syst Rev. 2012;11.
Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3.
Long L, Anderson L, Dewhirst AM, He J, Bridges C, Gandhi M, Taylor RS. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev. 2018;2.
Loughney LA, West MA, Kemp GJ, Grocott MPW, Jack S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst Rev. 2018;12.
Macedo LG, Saragiotto BT, Yamato TP, Costa LOP, Menezes Costa LC, Ostelo R, Maher CG. Motor control exercise for acute non-specific low back pain. Cochrane Database Syst Rev. 2016;2.
Macêdo TMF, Freitas DA, Chaves GSS, Holloway EA, Mendonça K. Breathing exercises for children with asthma. Cochrane Database Syst Rev. 2016;4.
Martin A, Booth JN, Laird Y, Sproule J, Reilly JJ, Saunders DH. Physical activity, diet and other behavioural interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. Cochrane Database Syst Rev. 2018;3.
McKeough ZJ, Velloso M, Lima VP, Alison JA. Upper limb exercise training for COPD. Cochrane Database Syst Rev. 2016;11.
McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Water-based exercise training for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;12.
McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;6.
Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, Olajide J, Mainardi GM, Corpeleijn E, O'Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6.
Meekums B, Karkou V, Nelson EA. Dance movement therapy for depression. Cochrane Database Syst Rev. 2015;2.
Meher S, Duley L. Exercise or other physical activity for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2006;2.
Mehrholz J, Kugler J, Pohl M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database Syst Rev. 2011;1.
Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;11.
Mehrholz J, Thomas S, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2017;8.
Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8.
Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8.
Montgomery P, Dennis JA. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002;4.
Morris NR, Kermeen FD, Holland AE. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev. 2017;1.
Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6.
Ngai SPC, Jones AYM, Tam WWS. Tai chi for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2016;6.
Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012;7.
O'Brien K, Nixon S, Glazier R, Tynan AM. Progressive resistive exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2004;4.
O'Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;8.
Østerås N, Kjeken I, Smedslund G, Moe RH, Slatkowsky-Christensen B, Uhlig T, Hagen KB. Exercise for hand osteoarthritis. Cochrane Database Syst Rev. 2017;1.
Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M, Buchbinder R. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database Syst Rev. 2014;8.
Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, Mrocki MA, Buchbinder R. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;6.
Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;6.
Panebianco M, Sridharan K, Ramaratnam S. Yoga for epilepsy. Cochrane Database Syst Rev. 2017;10.
Perry A, Lee SH, Cotton S, Kennedy C. Therapeutic exercises for affecting post-treatment swallowing in people treated for advanced-stage head and neck cancers. Cochrane Database Syst Rev. 2016;8.
Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev. 2017;11.
Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, Ravaud P. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;10.
Ren J, Xia J. Dance therapy for schizophrenia. Cochrane Database Syst Rev. 2013;10.
Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005;1.
Risom SS, Zwisler AD, Johansen PP, Sibilitz KL, Lindschou J, Gluud C, Taylor RS, Svendsen JH, Berg SK. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst Rev. 2017;2.
Romano M, Minozzi S, Bettany-Saltikov J, Zaina F, Chockalingam N, Kotwicki T, Maier-Hennes A, Negrini S. Exercises for adolescent idiopathic scoliosis. Cochrane Database Syst Rev. 2012;8.
Ryan JM, Cassidy EE, Noorduyn SG, O'Connell NE. Exercise interventions for cerebral palsy. Cochrane Database Syst Rev. 2017;6.
Saragiotto BT, Maher CG, Yamato TP, Costa LOP, Menezes Costa LC, Ostelo R, Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016;1.
Saunders DH, Sanderson M, Hayes S, Kilrane M, Greig CA, Brazzelli M, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2016;3.
Schulzke SM, Kaempfen S, Trachsel D, Patole SK. Physical activity programs for promoting bone mineralization and growth in preterm infants. Cochrane Database Syst Rev. 2014;4.
Seron P, Lanas F, Pardo Hernandez H, Bonfill Cosp X. Exercise for people with high cardiovascular risk. Cochrane Database Syst Rev. 2014;8.
Shaw KA, Gennat HC, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4.
Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;11.
Sibilitz KL, Berg SK, Tang LH, Risom SS, Gluud C, Lindschou J, Kober L, Hassager C, Taylor RS, Zwisler AD. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev. 2016;3.
Silva IS, Fregonezi GAF, Dias FAL, Ribeiro CTD, Guerra RO, Ferreira GMH. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;9.
States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev. 2009;3.
Strike K, Mulder K, Michael R. Exercise for haemophilia. Cochrane Database Syst Rev. 2016;12.
Takken T, Van Brussel M, Engelbert RH, van der Net JJ, Kuis W, Helders P. Exercise therapy in juvenile idiopathic arthritis. Cochrane Database Syst Rev. 2008;2.
Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, Lough F, Rees K, Singh SJ, Mordi IR. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4.
Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3.
Ussher MH, Taylor AH, Faulkner GEJ. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8.
Valentín-Gudiol M, Mattern-Baxter K, Girabent-Farrés M, Bagur-Calafat C, Hadders-Algra M, Angulo-Barroso RM. Treadmill interventions in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev. 2017;7.
van der Heijden RA, Lankhorst NE, van Linschoten R, Bierma-Zeinstra SMA, van Middelkoop M. Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst Rev. 2015;1.
Vloothuis JDM, Mulder M, Veerbeek JM, Konijnenbelt M, Visser-Meily JMA, Ket JCF, Kwakkel G, van Wegen EEH. Caregiver-mediated exercises for improving outcomes after stroke. Cochrane Database Syst Rev. 2016;12.
Voet NBM, van der Kooi EL. Riphagen, II, Lindeman E, van Engelen BGM, Geurts ACH: strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2013;7.
White CM, Pritchard J, Turner-Stokes L. Exercise for people with peripheral neuropathy. Cochrane Database Syst Rev. 2004;4.
Wieland LS, Skoetz N, Pilkington K, Vempati R, D'Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;1.
Williams AD, Bird ML, Hardcastle SGK, Kirschbaum M, Ogden KJ, Walters JAE. Exercise for reducing falls in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;10.
Williams MA, Srikesavan C, Heine PJ, Bruce J, Brosseau L, Hoxey-Thomas N, Lamb SE. Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev. 2018;7.
Yamamoto S, Hotta K, Ota E, Matsunaga A, Mori R. Exercise-based cardiac rehabilitation for people with implantable ventricular assist devices. Cochrane Database Syst Rev. 2018;9.
Yamato TP, Maher CG, Saragiotto BT, Hancock MJ, Ostelo R, Cabral CMN, Menezes Costa LC, Costa LOP. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7.
Yang ZY, Zhong HB, Mao C, Yuan JQ, Huang YF, Wu XY, Gao YM, Tang JL. Yoga for asthma. Cochrane Database Syst Rev. 2016;4.
Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015;4.
Zainuldin R, Mackey MG, Alison JA. Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;11.
Mok A, Khaw K-T, Luben R, Wareham N, Brage S. Physical activity trajectories and mortality: population based cohort study. Bmj. 2019;365:l2323.
Ekelund U, Brown WJ, Steene-Johannessen J, Fagerland MW, Owen N, Powell KE, Bauman AE, Lee IM. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br J Sports Med. 2019;53(14):886–94. https://doi.org/10.1136/bjsports-2017-098963 . Epub 2018 Jul 10.
Article PubMed Google Scholar
Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–10.
Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. The effect of physical activity on mortality and cardiovascular disease in 130000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54.
Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–95.
Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51.
Horton R. Offline: the gravy train of systematic reviews. Lancet. 2019;394(10211):1790.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):e1077–86.
Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72(14):1622–39.
Download references
Acknowledgements
Not applicable.
There was no funding source for this study. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Kleijnen Systematic Reviews Ltd., York, UK
Pawel Posadzki
Nanyang Technological University, Singapore, Singapore
Institute for Research in Operative Medicine, Witten/Herdecke University, Witten, Germany
Dawid Pieper, Nadja Könsgen & Annika Lena Neuhaus
School of Medicine, Keele University, Staffordshire, UK
Jozef Pilsudski University of Physical Education in Warsaw, Faculty Physical Education and Health, Biala Podlaska, Poland
Hubert Makaruk
Health Outcomes Division, University of Texas at Austin College of Pharmacy, Austin, USA
Monika Semwal
You can also search for this author in PubMed Google Scholar
Contributions
PP wrote the protocol, ran the searches, validated, analysed and synthesised data, wrote and revised the drafts. HM, NK and ALN screened and extracted data. MS and DP validated and analysed the data. RB ran statistical analyses. All authors contributed to writing and reviewing the manuscript. PP is the guarantor. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Dawid Pieper .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Supplementary Table 1. Main characteristics of included Cochrane systematic reviews evaluating the effects of physical activity/exercise on health outcomes ( n = 150). Supplementary Table 2. Additional information from Cochrane systematic reviews of the effects of physical activity/exercise on health outcomes ( n = 150). Supplementary Table 3. Conclusions from Cochrane systematic reviews “quote”. Supplementary Table 4 . AEs reported in Cochrane systematic reviews. Supplementary Table 5. Summary of withdrawals/non-adherence. Supplementary Table 6. Methodological quality assessment of the included Cochrane reviews with AMSTAR-2. Supplementary Table 7. Number of studies assessed as low risk of bias per domain. Supplementary Table 8. GRADE for the review’s main comparison. Supplementary Table 9. Studies reporting quality of life outcomes as mean difference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Posadzki, P., Pieper, D., Bajpai, R. et al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health 20 , 1724 (2020). https://doi.org/10.1186/s12889-020-09855-3
Download citation
Received : 01 April 2020
Accepted : 08 November 2020
Published : 16 November 2020
DOI : https://doi.org/10.1186/s12889-020-09855-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Health benefits of physical activity: a systematic review of current systematic reviews
Affiliation.
- 1 Physical Activity Promotion and Chronic Disease Prevention Unit, University of British Columbia, Vancouver, British Columbia, Canada.
- PMID: 28708630
- DOI: 10.1097/HCO.0000000000000437
Purpose of review: The health benefits of physical activity and exercise are clear; virtually everyone can benefit from becoming more physically active. Most international guidelines recommend a goal of 150 min/week of moderate-to-vigorous intensity physical activity. Many agencies have translated these recommendations to indicate that this volume of activity is the minimum required for health benefits. However, recent evidence has challenged this threshold-centered messaging as it may not be evidence-based and may create an unnecessary barrier to those who might benefit greatly from simply becoming more active. This systematic review evaluates recent systematic reviews that have examined the relationship between physical activity and health status.
Recent findings: Systematic reviews and/or meta-analyses (based largely on epidemiological studies consisting of large cohorts) have demonstrated a dose-response relationship between physical activity and premature mortality and the primary and secondary prevention of several chronic medical conditions. The relationships between physical activity and health outcomes are generally curvilinear such that marked health benefits are observed with relatively minor volumes of physical activity.
Summary: These findings challenge current threshold-based messaging related to physical activity and health. They emphasize that clinically relevant health benefits can be accrued by simply becoming more physically active. VIDEO ABSTRACT: http://links.lww.com/HCO/A42.
PubMed Disclaimer
Similar articles
- Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force [Internet]. Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS. Patnode CD, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. PMID: 29364620 Free Books & Documents. Review.
- Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Poitras VJ, Gray CE, Borghese MM, Carson V, Chaput JP, Janssen I, Katzmarzyk PT, Pate RR, Connor Gorber S, Kho ME, Sampson M, Tremblay MS. Poitras VJ, et al. Appl Physiol Nutr Metab. 2016 Jun;41(6 Suppl 3):S197-239. doi: 10.1139/apnm-2015-0663. Appl Physiol Nutr Metab. 2016. PMID: 27306431 Review.
- Reflections on Physical Activity and Health: What Should We Recommend? Warburton DE, Bredin SS. Warburton DE, et al. Can J Cardiol. 2016 Apr;32(4):495-504. doi: 10.1016/j.cjca.2016.01.024. Epub 2016 Mar 17. Can J Cardiol. 2016. PMID: 26995692 Review.
- The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. Spencer L, Rollo M, Hauck Y, MacDonald-Wicks L, Wood L, Hutchesson M, Giglia R, Smith R, Collins C. Spencer L, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):88-98. doi: 10.11124/jbisrir-2015-1812. JBI Database System Rev Implement Rep. 2015. PMID: 26447010
- [Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data]. Amato L, Colais P, Davoli M, Ferroni E, Fusco D, Minozzi S, Moirano F, Sciattella P, Vecchi S, Ventura M, Perucci CA. Amato L, et al. Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Review. Italian.
- Weekend physical activity profiles and their relationship with quality of life: The SOPHYA cohort of Swiss children and adolescents. Darkhawaja R, Hänggi J, Bringolf-Isler B, Kayser B, Suggs LS, Kwiatkowski M, Probst-Hensch N. Darkhawaja R, et al. PLoS One. 2024 May 31;19(5):e0298890. doi: 10.1371/journal.pone.0298890. eCollection 2024. PLoS One. 2024. PMID: 38820541 Free PMC article.
- A mixed-method analysis of the contribution of informal sport to public health in Australia. Jeanes R, O'Connor J, Penney D, Spaaij R, Magee J, O' Hara E, Lymbery L. Jeanes R, et al. Health Promot Int. 2024 Jun 1;39(3):daae048. doi: 10.1093/heapro/daae048. Health Promot Int. 2024. PMID: 38809233 Free PMC article.
- Beyond body mass index: a synthesis of lifestyle factors that may influence in vitro fertilisation outcomes. Schneider E, Hamer O, Smith C, Hill J. Schneider E, et al. Br J Midwifery. 2023 Aug 2;31(8):436-444. doi: 10.12968/bjom.2023.31.8.436. Epub 2023 Aug 4. Br J Midwifery. 2023. PMID: 38808077 Free PMC article. No abstract available.
- The 2023 Latin America report of the Lancet Countdown on health and climate change: the imperative for health-centred climate-resilient development. Hartinger SM, Palmeiro-Silva YK, Llerena-Cayo C, Blanco-Villafuerte L, Escobar LE, Diaz A, Sarmiento JH, Lescano AG, Melo O, Rojas-Rueda D, Takahashi B, Callaghan M, Chesini F, Dasgupta S, Posse CG, Gouveia N, Martins de Carvalho A, Miranda-Chacón Z, Mohajeri N, Pantoja C, Robinson EJZ, Salas MF, Santiago R, Sauma E, Santos-Vega M, Scamman D, Sergeeva M, Souza de Camargo T, Sorensen C, Umaña JD, Yglesias-González M, Walawender M, Buss D, Romanello M. Hartinger SM, et al. Lancet Reg Health Am. 2024 Apr 23;33:100746. doi: 10.1016/j.lana.2024.100746. eCollection 2024 May. Lancet Reg Health Am. 2024. PMID: 38800647 Free PMC article. Review.
- Fundamental Stability Skills: Reliability Analysis Using the Alfamov Assessment Tool. Santos-Miranda E, Carballo-Fazanes A, Rey E, Piñeiro-García-Tuñón I, Abelairas-Gómez C. Santos-Miranda E, et al. Children (Basel). 2024 May 11;11(5):583. doi: 10.3390/children11050583. Children (Basel). 2024. PMID: 38790579 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Ingenta plc
- Ovid Technologies, Inc.
- Wolters Kluwer
Other Literature Sources
- scite Smart Citations
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 January 2023
Exercising is good for the brain but exercising outside is potentially better
- Katherine Boere 1 ,
- Kelsey Lloyd 1 ,
- Gordon Binsted 2 &
- Olave E. Krigolson 1
Scientific Reports volume 13 , Article number: 1140 ( 2023 ) Cite this article
29k Accesses
8 Citations
820 Altmetric
Metrics details
- Cognitive control
- Health policy
- Human behaviour
- Neuroscience
It is well known that exercise increases cognitive function. However, the environment in which the exercise is performed may be just as important as the exercise itself. Time spent in natural outdoor environments has been found to lead to increases in cognition similar to those resulting from acute exercise. Therefore, the benefits of both exercise and nature exposure suggest an additive impact on brain function when both factors are combined. This raises the question: what is the interaction between acute exercise and environment on cognition? We answered this question using electroencephalography to probe cognitive function using the oddball task before and after brief indoor and outdoor walks on 30 participants (average 21 years old, 95% CI [20, 22]). Our results demonstrate improved performance and an increase in the amplitude of the P300, an event-related neural response commonly associated with attention and working memory, following a 15-min walk outside; a result not seen following a 15-min walk inside. Importantly, this finding indicates that the environment may play a more substantial role in increasing cognitive function such as attention than exercise, at least in terms of acute exercise (i.e., a brief walk). With the world’s growing urbanization and the associated increase in sedentary time indoors, a deeper understanding of how these factors interact and influence cognition may be critical to combat adverse health effects.
Similar content being viewed by others

Microdosing with psilocybin mushrooms: a double-blind placebo-controlled study

Single dose creatine improves cognitive performance and induces changes in cerebral high energy phosphates during sleep deprivation

Cognitive control training with domain-general response inhibition does not change children’s brains or behavior
Introduction.
It is well known that exercise generally enhances cognitive function 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 . However, the environment in which exercise is performed may be just as important as the exercise itself 11 , 12 , 13 . A growing body of research highlights nature's positive impacts on improving cognition and mental health 14 , 15 , 16 . Thus, with considerable evidence to support the benefits of exercise and being in nature, it is logical to expect that combining these two factors would lead to an ever-greater overall increase in cognition. Indeed, evidence supports this supposition: exercising outdoors in natural environments produces more benefits to the brain than exercising indoors 12 , 17 , 18 , 19 , 20 , 21 , 22 , 23 . Yet the findings are unclear for acute exercise durations under 20 min. This raises the question: How does the environment influence cognitive function for a brief exercise period? Gaining a deeper understanding of these underlying influences on the brain is important to combat the world’s growing urbanization and the associated increase in the amount of time spent indoors 24 .
In terms of cognition, acute outdoor exercise has primarily been found to enhance executive functions dependent on the prefrontal cortex, such as attention, working memory, and inhibitory control 1 , 3 . For instance, Bailey and colleagues 19 found that participants who walked in an outdoor natural environment performed significantly better on a cognitive task—the Stroop task—than those who walked inside. These findings are further supported by Berman et al. 20 , who had participants complete a different cognitive task (the backwards digit span task) before and after a 35-min walk in a forest or an urban environment. In a key manipulation, the researchers induced cognitive fatigue in participants by having them complete a rigorous memory task before walking. Berman and colleagues’ results showed that on the cognitive task performed after the walk, participants who walked in the forest performed better than those who had walked in an urban environment suggesting that nature played an essential role in restoring the cognitive resources that were depleted by the memory task 20 . Together, these results provide evidence that outdoor exercise enhances executive function to a greater extent than indoor exercise.
Neuroimaging has provided key insight into the impact of acute exercise on the brain. Research with functional magnetic resonance imaging and functional near-infrared spectroscopy (fNIRS) has demonstrated that acute exercise drives improvements in cognition via increased cerebral blood flow (CBF) to the prefrontal cortex 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 . For example, Yanagisawa and colleagues used fNIRS to examine brain regions activated through acute exercise-induced enhancements in cognitive performance 27 . The researchers found that acute moderate exercise improved performance on the Stroop task and elicited increased activation in the dorsolateral prefrontal cortex- a region explicitly associated with executive function 27 . In addition, a range of neurotransmitters have been implicated in acute exercise's signalling pathways that induce positive cognitive and mood effects 1 , 3 , 28 , 29 , 30 , 31 , 32 . Specifically, several studies have found increases in dopamine, epinephrine, and norepinephrine in the prefrontal cortex post-exercise; all indicated to be involved in neuromodulating behaviours such as attention, reward, learning, and memory 29 , 30 , 31 , 32 , 33 . Collectively, these findings highlight the impact of acute exercise on the prefrontal cortex and indicate several mechanisms by which acute exercise influences the neurophysiology of this process.
Here we used neuroimaging to investigate the interaction of brief exercise and environment on cognition. Specifically, we utilized mobile electroencephalography (mEEG) to measure indices of cognitive performance prior to and after brief 15-min indoor and outdoor walks. Before and after each walk, participants completed a standard visual oddball task while mEEG data was recorded. Based on the abundance of literature indicating that exercise enhances cognitive performance 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , we hypothesized that we would see an increase in the amplitude of the P300—a component of the human event-related brain potential (ERP) associated with working memory and attention—following exercise. Additionally, given the well-documented positive effects of nature on the brain 14 , 15 , 16 , we further hypothesized that the increase in the amplitude of the P300 would be greater following exercise outside than the following exercise inside.
To investigate the impact of walking location, we conducted a two (location: inside versus outside) by two (time: pre-test versus post-test) fully repeated measures analysis of variance on reaction time, accuracy, and P300 amplitude. See Table 1 for a summary of results.
Reaction time
An analysis of reaction time revealed a main effect for time (F(1,29) = 11.01, p = 0.002, η 2 = 0.275) that showed that reaction decreased slightly following 15 min of walking (see Fig. 1 ). This was qualified by an interaction between walking location and time (F(1,29) = 3.95, p = 0.05, η 2 = − 0.120). Decomposition of the interaction revealed that reaction time decreased after a 15 min outdoor walk (t(29) = 5.07, p = 0.000, d = 0.926) but not following a 15 min indoor walk (t(29) = 0.84, p = 0.409, d = 0.152). We did not find a main effect for walking location (p > 0.05).
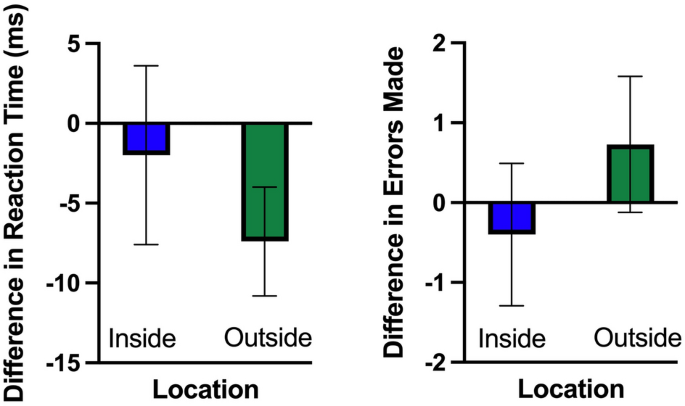
The difference in reaction time and accuracy on the oddball task between pre- and post- indoor and outdoor walk. Value on plot = post walk score − pre-walk score . Therefore, negative values indicate improved performance. Error bars reflect 95% within-subject confidence intervals.
Our analysis of our accuracy data (number of errors made) revealed no main effects or interactions (F(1,29) = 3.59, p > 0.05, η 2 = 0.10, see Fig. 1 ).
P300 amplitude
Here, we found a main effect of time (F(1,29) = 12.40, p = 0.001, η 2 = 0.299) that revealed an increase in P300 amplitude following 15 min of walking. This was qualified by an interaction between walking location and time (F(1,29) = 5.62, p = 0.025, η 2 = 0.162). Decomposition of that interaction revealed no change in P300 amplitude following an indoor walk (t(29) = 0.23, p = 0.82, d = 0.032) but an increase in P300 amplitude following an outdoor walk (t(29) = 4.49, p < 0.001, d = 0.434, see Figs. 2 and 3 ). We did not find a main effect for walking location (p > 0.05).
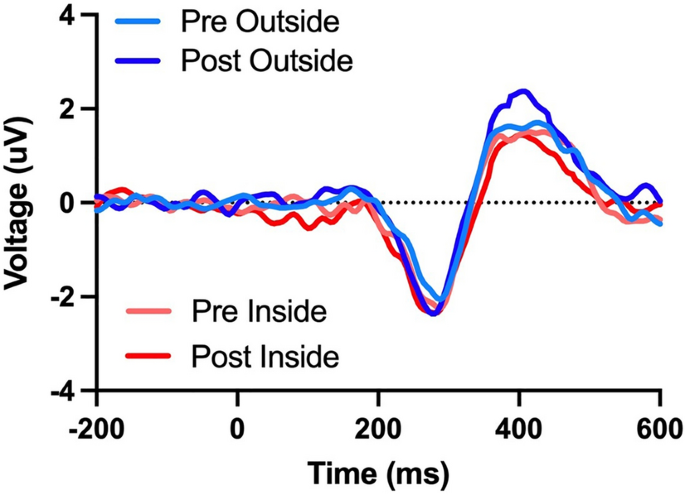
Grand average ERP difference waveforms for the visual oddball pre-test and post-test for indoor and outdoor walks. Note the P300 ERP component increased in the post-test following outdoor walks.
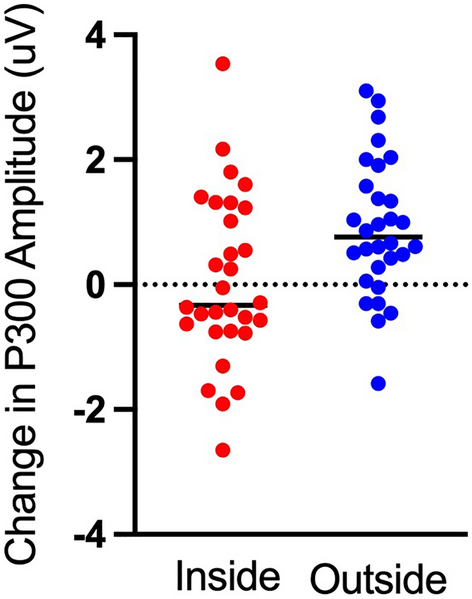
The difference in P300 peak amplitude between the post-test and pre-test for indoor and outdoor walks. Individual data are plotted for each participant.
In the present study, we examined how the walking environment—indoors or outdoors—interacted with acute exercise to impact cognitive function, specifically the oddball task and P300 amplitude. As predicted, exercise enhanced our measures of cognition, as evidenced by the rise in the amplitude of the P300 ERP component. However, this only occurred when the exercise took place outdoors. The main effect of exercise was extended to the behavioural results for reaction time, showing an overall decrease in reaction time post-walk. Yet again, the decrease in reaction times only occurred for outdoor walks. No difference was found in accuracy between groups, and we propose this is due to the ease of task difficulty. Our result is broadly consistent with a large body of research demonstrating that acute exercise enhances cognitive performance 1 , 2 , 3 , 5 , 6 , 7 , 9 , 10 . Further, previous EEG studies have shown that acute exercise increases P300 amplitude during task performance—a result associated with enhanced attentional processes and working memory in the pre-frontal cortex 34 , 35 , 36 , 37 , 38 . Our results align with notable findings that acute exercise improves brain function and is further supported by the positive effects found on task performance.
More importantly, we also found that outdoor exercise had an additional impact on cognitive attentional scores. Specifically, we found that the amplitude of the P300 ERP component was greater following a walk outside relative to a walk inside. In addition, reaction times were lower for outdoor walks than for indoor ones. This result is consistent with both attention restoration theory 13 , 39 , 40 , 41 and previous findings supporting the idea that natural environments facilitate attention restoration during acute exercise 14 , 15 , 16 , 42 . The attentional restoration theory proposed by Kaplan 39 posits that natural environments provide a sense of "being away" from routines and inducing "soft fascination." Rather than dominating one's attention, nature restores mental capacities and enhances cognitive performance 41 , 42 . Further, Kaplan and Berman 20 proposed that natural environments restore directed attention, a shared resource that supports executive function in the prefrontal cortex.
Neurophysiologically, attention can be described as increased activity in a particular brain area involved in processing stimuli. As per the hemodynamic response, we assume increased activation signifies increased CBF in that specific brain region. For reference, the hemodynamic response is a homeostatic mechanism that replenishes the nutrients used by biological tissues by adjusting blood flow to areas of activity. Keeping in mind that this fresh, nutrient-rich blood is a limited resource—the attentional restoration theory implies that exposure to natural environments restores this mechanism by reducing unrequired increases in CBF. Regarding exercise’s effect on CBF, the reported results are mixed. We believe this is due to the amount of variability within exercise-based studies on the brain—as intensity, duration, and fitness are all significant factors that could influence the rate of CBF. However, when examining the effect of acute low-moderate intensity exercise , we identified abundant literature supporting that exercise increases CBF 1 , 2 , 5 , 7 , 25 , 26 , 27 , 28 . Indeed, with the lungs working harder during exercise, this oxygen surplus must be transported through the circulatory system. To facilitate this process, the body responds with a rise in heart rate and a widening of the arterial walls, thereby increasing blood flow globally, including to the brain. Taken together, we suggest that exercise increases CBF while the natural outdoor environments reduce and restore CBF mechanisms. Therefore, post-exercise and nature exposure, the effect of reduced blood flow to brain areas focused on irrelevant stimuli (brain areas not currently required) concurrently allows for increased blood flow and activation in areas pertinent to important stimuli (i.e., the task at hand).
An intriguing finding in our study is that we did not see a specific increase in the measured index of cognitive function following a brief walk indoors (i.e., we did not see an increase in P300 amplitude), nor did we find an increase in cognitive performance. This result conflicts with a significant meta-analysis by Chang and colleagues 3 and fails to support the previously stated inference that brief exercise (less than 20 min) could promote cognitive function. The results imply that environmental location may facilitate attention restoration and improve indices of cognition without exercise. Such impacts of both nature and acute exercise could be synergistic; however, this begs the question, which plays a more prominent role in improving cognition? Although we cannot conclude this without separating exercise and environment, our results point to a more substantial role of environment in increasing cognitive function. Our findings suggest that if one has only 15 min to exercise 24 , performing it outdoors appears to have a greater effect on ERP indices of attention and working memory than indoors.
Notably, there are limitations to the conclusions we can draw from our findings. First, there is a lack of objective exercise intensity measurement. Although the participants were clearly explained, demonstrated, and paced with the desired walking intensity, no heart rate monitor was used. We recommend that all future studies introduce heart rate monitoring and rate of perceived exertion to further control variability and increase the validity of results. Second, previous research suggests that exercise duration must be over 20 min to affect cognitive performance positively 1 , 3 , 6 . Here, the duration of the walking protocol was decided from preliminary pilot testing, which recorded the time it would take a student to walk at a low/moderate intensity around a set track of 2 km. This resulted in a walking protocol of roughly 15 min. In addition, we specifically sought to investigate if the effects of this exercise differ when undertaken outside. Indeed, this time constraint may be why we did not find significant exercise-induced cognitive improvements. However, from this perspective, if the exercise duration is not long enough to influence cognitive performance, it is reasonable to deduce that the remaining variable—the environment—may be the driving force in increasing P300 amplitudes.
Considering the constraints of using mEEG over traditional large array systems is also essential. On mEEG, electrodes are placed in non-standard positions for observing “classic” ERP components such as the P300. Offline analyses of the oddball task typically would have focused on a central line electrode Pz as opposed to TP9 and TP10. Due to the MUSE device not having an electrode at Pz, we were forced to make this adaption. However, this is not a significant concern as previous work in our laboratory has demonstrated that although this produces a slightly different waveform, it still reveals clearly observable and quantifiable ERP components 47 , 48 . Second, due to time lags inherent with Bluetooth data connection and the inability to insert experimental event markers, we know there is a notable “jitter” in the EEG data. Yet, our previous work demonstrates that this does not prevent the recording or quantification of ERP components—it simply adds a delay which is accounted for in our analysis 47 , 48 .
In conclusion, we demonstrate that a brief walk outside results in a greater increase in cognitive function than a short walk inside. Given the continued growth in urbanization and a move to an indoor lifestyle, our results highlight the importance of spending time in nature, especially when exercising. Indeed, in a world where many people “hit the gym” before or after work or on their lunch break, our results suggest that these people would be better served by simply “getting outside”.
Study approval
All participants gave their informed written consent, approved by the Human Research Ethics Board at the University of Victoria (HREB: BC17-456). Human Research Ethics Board approved all experimental protocols at the University of Victoria (HREB: BC17-456). The experiment conformed to the ethical standards prescribed by the 1964 Declaration of Helsinki and subsequent revisions. All participants were given a comprehensive set of instructions regarding the procedure and tests, agreed both verbally and in the consent form to the testing procedures, and were given course credit in exchange for their participation.
Participants
Participants in the present study were undergraduate, and graduate students at the University of Victoria, British Columbia, Canada (n = 32). Previous work in our laboratory 43 conducted an ERP experiment with a sample size of 500 and found that detecting an ERP elicits a large effect size of 0.8, following recommendations from Cohen 44 . We conducted a power analysis for a repeated measures t test using this standardized effect size, an alpha of 0.05, and the desired power of 0.95, which yielded a prospective sample size of 25 participants. Moreover, our laboratory follows a protocol wherein ERP studies include a minimum of 30 participants, corresponding to a power of 0.99. It is important to note that participants with more than 50% of their data discarded due to excessive EEG artifacts were not included in our analysis. To avoid conducting underpowered research 45 , we kept testing participants until we had achieved our a priori set size of 30. Thus, the data presented here reflect the first 30 participants who met our EEG artifact criterion and resulted in the removal of two participants (n = 30; 21 females, average age: 21, 95% CI [20, 22]) Participants were asked to refrain from exercising and consuming alcohol for 24 h before testing. Further, each participant was instructed to abstain from eating or consuming caffeine two hours before testing. All participants had normal or corrected-to-normal vision and no known neurological impairments.
Apparatus and procedure
Participants completed a standard visual oddball task on an Apple iPad (Apple Inc., Cupertino, CA, USA). At the same time, EEG data were recorded from a 2016 Muse EEG system (Interaxon Inc., Toronto, ON, Canada). The task and recordings were collected prior to and after 15-min indoor and outdoor walks. Walks were conducted on two separate days, on average 2 days apart and no more than 1 week apart (range = 1–7 days). The first participant walked inside on day one and outside on day 2. Subsequent participants alternated the walk locations on a participant-to-participant basis. The study was held at one of three times: 10:15 am, 12 pm or 1:45 pm—in an effort to limit the effects of the daily circadian rhythm.
Here, we choose the oddball task, rather than the previously used Stroop task 19 , 20 , to expand the current scientific literature on this topic. Both tasks are highly well-known in cognitive neuroscience, and both have been previously used to measure selective attention capacity and working memory 1 , 2 , 3 , 4 , 19 , 36 , 38 . Therefore finding the same results with a different yet equally valid task would only provide additional power to the result. During performance of the oddball task, participants saw a series of blue (RGB value = [0, 0, 255]) and green (RGB value = [0, 255, 0]) colored circles that appeared for 800 ms in the center of a dark gray background (RGB value = [108, 108, 108]) on the iPad screen. Before the onset of the first circle and between the presentation of subsequent circles, a black (RGB value = [0, 0, 0]) fixation cross was presented for 200 to 500 ms. Participants were not told the frequency of the blue and green circles: blue appeared less frequently (oddball: 30%) than green circles (control: 70%). Stimulus order was randomized, with the constraint that the stimulus presentation software ensured that no more than two infrequent (oddball) circles appeared consecutively. Participants were instructed to quickly press the bottom left or right of the iPad screen when they saw one of the blue circles (oddball) and not respond when they saw the green circles (control). The circles were presented for 800–1200 ms, and the trial ended automatically on oddball trials in which the participants did not respond. Each trial began with a fixation cross as soon as the previous circle disappeared. Participants completed four blocks of 100 trials.
After completing the pre-walk oddball task, participants completed a brief walk inside the Engineering Lab Building or outside the Alumni Chip Trail at the University of Victoria. This trail was a green and lush forested path around the campus. For both locations, the distance of the walk was 2 km. Participants were asked to walk at their normal pace—above leisurely but not hard breathing. For this reason, participants’ walk times varied between 14 and 17 min, with an average of 15 min. In addition, participants were asked not to speak to anyone, use their cell phones or listen to music for the entirety of the walk. A research assistant timed each walk and walked approximately 10 m behind the participant, pacing them to ensure they maintained the same walking intensity. Aside from the experimenter's directional instructions to ensure the participant kept a set pace, there was no conversation between the experimenter and the participant. After completing the walk, participants completed the oddball task again. All EEG recording was done in a quiet room within the Engineering Lab Wing building.
Data acquisition
EEG data were recorded from a Muse EEG headband (Muse Version: 2016) sampling at 256 Hz (see http://www.choosemuse.com for full technical specifications, see Fig. 4 ). The Muse EEG system has electrodes located analogously to FPz, AF7, AF8, TP9, and TP10, with FPz utilized as the reference electrode during recording. EEG data were recorded, and the visual oddball task was performed with the PEER iOS application ( https://brainwavesoftware.ca/ ). Note that the PEER application does not send event markers to the EEG headset as per a traditional ERP study 46 but instead reads the EEG data with known Bluetooth lag and jitter. Specifically, we have written MATLAB code that allows two-way communication via the OSC protocol to send markers to mark a continuous EEG recording. As such, we “marked” the EEG data at the exact onset time when the circles were drawn. Due to the Bluetooth lag, the EEG samples corresponding to this point in time did not arrive for 18–20 ms on average with a jitter of approximately 5 ms 47 , 48 . It is important to note that this jitter only impacted the initial signal locking between the MUSE system and our software and did not vary over time. In addition, the random delays in the temporal onset of our data collection would have a Gaussian distribution, and thus the lags would average out.
The 2016 Muse EEG system was made by InterAxon Inc with electrodes labelled AF7, AF8, TP9, and TP10. Reference electrode is labelled at FPz.
Further, while the timing onset via Bluetooth is not “guaranteed,”—the order of data packets is ( https://www.bluetooth.com/ ). Simply put, this means that all data points are guaranteed to be in the same order they were continuously collected in. As such, the averaging of temporal onset did not impact the present data as much as one might assume. Thus, the signal did not differ from trial to trial but from participant to participant. Signal quality was then inferred by examining the variance per second on each EEG channel, and data collection began when all channels had a variance per second less than 200. Finally, we have previously shown that this method still results in a reliable albeit diminished ERP response 47 , 48 (see these papers for full details on Bluetooth timing issues and delay/jitter data).
Data processing and analysis
Data were processed offline in MATLAB using EEGLAB 49 and custom code. We did not re-reference the continuous EEG data offline as our ERP analysis focused on the two posterior Muse electrodes (TP9 and TP10) referenced when recording electrode FPz. Continuous EEG data were filtered with a dual-pass Butterworth filter with a 0.1–30 Hz passband and a 60 Hz notch filter. A preliminary analysis of the data revealed no lateralized effects; further, we wanted to improve the signal-to-noise ratio of the ERP measures 50 , so we created a pooled frontal and a pooled posterior virtual electrode by averaging across the frontal (AF7 and AF8) and the rear (TP9 and TP10) electrodes, respectively. Our ERP analysis only focused on the new average posterior virtual electrode based on our previous work 47 , 48 . We also chose not to analyze the ERP effects for the frontal ERP channels as re-referencing the EEG data mirrors the components given the high correlation between the Muse EEG channels—see https://www.krigolsonlab.com/muse-research.html for exploratory analyses examining this issue that provided the rationale for the choices we made here.
After filtering, epochs of data from 200 ms before to 600 ms after stimulus onset (oddball, control) were extracted from the continuous EEG data. Segments were then baseline corrected using the 200 ms preceding stimulus onset. An artifact rejection algorithm was then implemented to remove noise and blink artifacts; this procedure discarded segments with an absolute difference of more than 60 μV (on average: 30% [28.5, 31.5]). Segments were then averaged for each participant's oddball and control trials, and a difference waveform was constructed by subtracting the average control from the average oddball ERP waveform. Grand average ERPs were generated by averaging all conditional (oddball, control) and difference waveforms for each participant and the peak component latencies were identified (N200: ~ 200 ms; P300: ~ 400 ms). N200 and P300 ERP component amplitudes and latencies were quantified at the participant level by finding the local minimal (N200: 150–250 ms) and local maximal (P300: 400–600 ms) voltage amplitudes within the windows mentioned above around the grand average component peaks. ERP peak amplitude data were statistically analyzed using a two (walk location: indoor, outdoor) by two (time: pre-test, post-test) fully repeated measures analysis of variance. Post-hoc decomposition of the interaction was done via dependent samples t tests. Reaction time was calculated as the time it took participants to press the screen after the stimulus circle was presented. Accuracy was defined by the number of errors made during the task (responses to control stimulus or no response to oddball stimulus). Finally, mean data for ERP and behavioural data are presented with 95% confidence intervals.
Data availability
All processing scripts can be found at https://github.com/Neuro-Tools . The data that supports the findings of this study are available from https://osf.io/5m24j/ .
Basso, J. C. & Suzuki, W. A. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plasticity 2 , 127–152 (2017).
Article Google Scholar
Brisswalter, J., Collardeau, M. & Ren, A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 32 , 555–566 (2002).
Chang, Y. K., Labban, J. D., Gapin, J. I. & Etnier, J. L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 1453 , 87–101 (2012).
Article CAS Google Scholar
Chang, Y.-K., Pesce, C., Chiang, Y.-T., Kuo, C.-Y. & Fong, D.-Y. Antecedent acute cycling exercise affects attention control: An ERP study using attention network test. Front. Hum. Neurosci. 9 , 156 (2015).
Hillman, C. H., Erickson, K. I. & Kramer, A. F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 9 , 58–65 (2008).
Lambourne, K. & Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 1341 , 12–24 (2010).
Ludyga, S., Gerber, M., Brand, S., Holsboer-Trachsler, E. & Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 53 , 1611–1626 (2016).
McMorris, T. & Hale, B. J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: A meta-analytical investigation. Brain Cogn. 80 , 338–351 (2012).
Sibley, B. A., Etnier, J. L. & Le Masurier, G. C. Effects of an acute bout of exercise on cognitive aspects of Stroop performance. J. Sport Exerc. Psychol. 28 , 285–299 (2006).
Tomporowski, P. D. Effects of acute bouts of exercise on cognition. Acta Physiol. (Oxf) 112 , 297–324 (2003).
Google Scholar
Lahart, I., Darcy, P., Gidlow, C. & Calogiuri, G. The effects of green exercise on physical and mental wellbeing: A systematic review. Int. J. Environ. Res. Public Health 16 , 1352 (2019).
Pretty, J., Peacock, J., Sellens, M. & Griffin, M. The mental and physical health outcomes of green exercise. Int. J. Environ. Health Res. 15 , 319–337 (2005).
Thompson Coon, J. et al. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A systematic review. Environ. Sci. Technol. 45 , 1761–1772 (2011).
Article ADS CAS Google Scholar
Berto, R. Exposure to restorative environments helps restore attentional capacity. J. Environ. Psychol. 25 , 249–259 (2005).
Bowler, D. E., Buyung-Ali, L. M., Knight, T. M. & Pullin, A. S. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health 10 , 456 (2010).
Bratman, G. N., Daily, G. C., Levy, B. J. & Gross, J. J. The benefits of nature experience: Improved affect and cognition. Landsc. Urban Plan. 138 , 41–50 (2015).
Araújo, D., Brymer, E., Brito, H., Withagen, R. & Davids, K. The empowering variability of affordances of nature: Why do exercisers feel better after performing the same exercise in natural environments than in indoor environments?. Psychol. Sport Exerc. 42 , 138–145 (2019).
Aspinall, P., Mavros, P., Coyne, R. & Roe, J. The urban brain: Analysing outdoor physical activity with mobile EEG. Br. J. Sports Med. 49 , 272–276 (2013).
Bailey, A. W., Allen, G., Herndon, J. & Demastus, C. Cognitive benefits of walking in natural versus built environments. World Leisure J. 60 , 293–305 (2018).
Berman, M. G., Jonides, J. & Kaplan, S. The cognitive benefits of interacting with nature. Psychol. Sci. 19 , 1207–1212 (2008).
Brito, H. S. et al. Benefits to performance and well-being of nature-based exercise: A critical systematic review and meta-analysis. Environ. Sci. Technol. 56 , 62–77 (2021).
Article ADS Google Scholar
Gidlow, C. J. et al. Where to put your best foot forward: Psycho-physiological responses to walking in natural and urban environments. J. Environ. Psychol. 45 , 22–29 (2016).
Rogerson, M. & Barton, J. Effects of the visual exercise environments on cognitive directed attention, energy expenditure and perceived exertion. Int. J. Environ. Res. Public Health 12 , 7321–7336 (2015).
Prince, S. A., Reed, J. L., McFetridge, C., Tremblay, M. S. & Reid, R. D. Correlates of sedentary behaviour in adults: A systematic review. Obes. Rev. 18 , 915–935 (2017).
Byun, K. et al. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage 98 , 336–345 (2014).
Hyodo, K. et al. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol. Aging 33 , 2621–2632 (2012).
Yanagisawa, H. et al. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 50 , 1702–1710 (2010).
Dalsgaard, M. K. et al. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J. Physiol. 554 , 571–578 (2004).
McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 165 , 291–299 (2016).
Skriver, K. et al. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 116 , 46–58 (2014).
Winter, B. et al. High impact running improves learning. Neurobiol. Learn. Mem. 87 , 597–609 (2007).
Kashihara, K., Maruyama, T., Murota, M. & Nakahara, Y. Positive effects of acute and moderate physical exercise on cognitive function. J. Physiol. Anthropol. 28 , 155–164 (2009).
Ranjbar-Slamloo, Y. & Fazlali, Z. Dopamine and noradrenaline in the brain; Overlapping or dissociate functions?. Front. Mol. Neurosci. 12 , 334 (2020).
Chang, Y.-K. et al. Acute exercise has a general facilitative effect on cognitive function: A combined ERP temporal dynamics and BDNF study. Psychophysiology 54 , 289–300 (2016).
Hillman, C. H., Snook, E. M. & Jerome, G. J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 48 , 307–314 (2003).
Hillman, C. H. et al. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 159 , 1044–1054 (2009).
Kamijo, K. et al. Differential influences of exercise intensity on information processing in the central nervous system. Eur. J. Appl. Physiol. 92 , 305–311 (2004).
Kamijo, K., Nishihira, Y., Garshiura, T. & Kuroiwa, K. The interactive effect of exercise intensity and task difficulty on human cognitive processing. Int. J. Psychophysiol. 65 , 114–121 (2007).
Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 15 , 169–182 (1995).
Stevenson, M. P., Schilhab, T. & Bentsen, P. Attention Restoration Theory II: A systematic review to clarify attention processes affected by exposure to natural environments. J. Toxicol. Environ. Health Part B 21 , 227–268 (2018).
Basu, A., Duvall, J. & Kaplan, R. Attention restoration theory: Exploring the role of soft fascination and mental bandwidth. Environ. Behav. 51 , 1055–1081 (2018).
McMahan, E. A. & Estes, D. The effect of contact with natural environments on positive and negative affect: A meta-analysis. J. Posit. Psychol. 10 , 507–519 (2015).
Williams, C. C., Ferguson, T. D., Hassall, C. D., Abimbola, W. & Krigolson, O. E. The ERP, frequency, and time–frequency correlates of feedback processing: Insights from a large sample study. Psychophysiology 58 , e13722 (2020).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (L. Erlbaum Associates, 1988).
MATH Google Scholar
Ioannidis, J. P. A. Why most published research findings are false. PLoS Med. 2 , e124 (2005).
Luck, S. J. An Introduction to the Event-Related Potential Technique (MIT Press, 2014).
Krigolson, O. E., Williams, C. C., Norton, A., Hassall, C. D. & Colino, F. L. Choosing MUSE: Validation of a low-cost, portable EEG system for ERP research. Front. Neurosci. 11 , 109 (2017).
Krigolson, O. E. et al. Using muse: Rapid mobile assessment of brain performance. Front. Neurosci. 15 , 634147 (2021).
Delorme, A. & Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134 , 9–21 (2004).
Oken, B. S. & Chiappa, K. H. Statistical issues concerning computerized analysis of brainwave topography. Ann. Neurol. 19 , 493–494 (1986).
Download references
Acknowledgements
This research was supported by NSERC Discovery Grant (RGPIN 2016-0943).
Author information
Authors and affiliations.
Theoretical and Applied Neuroscience Laboratory, University of Victoria, STN CSC, PO Box 1700, Victoria, BC, V8W 2Y2, Canada
Katherine Boere, Kelsey Lloyd & Olave E. Krigolson
Faculty of Health, York University, 4700 Keele St, Toronto, ON, M3J 1P3, Canada
Gordon Binsted
You can also search for this author in PubMed Google Scholar
Contributions
K.B. designed the study, wrote the manuscript, and analyzed the data. K.L. was responsible for data collection, assisted with data analysis, and helped with manuscript preparation. G.B. and O.E.K. directed the research, supported data analysis, and facilitated the writing of the final version of the manuscript.
Corresponding author
Correspondence to Katherine Boere .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Boere, K., Lloyd, K., Binsted, G. et al. Exercising is good for the brain but exercising outside is potentially better. Sci Rep 13 , 1140 (2023). https://doi.org/10.1038/s41598-022-26093-2
Download citation
Received : 27 May 2022
Accepted : 09 December 2022
Published : 20 January 2023
DOI : https://doi.org/10.1038/s41598-022-26093-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Physical Activity Is Good for the Mind and the Body

Health and Well-Being Matter is the monthly blog of the Director of the Office of Disease Prevention and Health Promotion.
Everyone has their own way to “recharge” their sense of well-being — something that makes them feel good physically, emotionally, and spiritually even if they aren’t consciously aware of it. Personally, I know that few things can improve my day as quickly as a walk around the block or even just getting up from my desk and doing some push-ups. A hike through the woods is ideal when I can make it happen. But that’s me. It’s not simply that I enjoy these activities but also that they literally make me feel better and clear my mind.
Mental health and physical health are closely connected. No kidding — what’s good for the body is often good for the mind. Knowing what you can do physically that has this effect for you will change your day and your life.
Physical activity has many well-established mental health benefits. These are published in the Physical Activity Guidelines for Americans and include improved brain health and cognitive function (the ability to think, if you will), a reduced risk of anxiety and depression, and improved sleep and overall quality of life. Although not a cure-all, increasing physical activity directly contributes to improved mental health and better overall health and well-being.
Learning how to routinely manage stress and getting screened for depression are simply good prevention practices. Awareness is especially critical at this time of year when disruptions to healthy habits and choices can be more likely and more jarring. Shorter days and colder temperatures have a way of interrupting routines — as do the holidays, with both their joys and their stresses. When the plentiful sunshine and clear skies of temperate months give way to unpredictable weather, less daylight, and festive gatherings, it may happen unconsciously or seem natural to be distracted from being as physically active. However, that tendency is precisely why it’s so important that we are ever more mindful of our physical and emotional health — and how we can maintain both — during this time of year.
Roughly half of all people in the United States will be diagnosed with a mental health disorder at some point in their lifetime, with anxiety and anxiety disorders being the most common. Major depression, another of the most common mental health disorders, is also a leading cause of disability for middle-aged adults. Compounding all of this, mental health disorders like depression and anxiety can affect people’s ability to take part in health-promoting behaviors, including physical activity. In addition, physical health problems can contribute to mental health problems and make it harder for people to get treatment for mental health disorders.
The COVID-19 pandemic has brought the need to take care of our physical and emotional health to light even more so these past 2 years. Recently, the U.S. Surgeon General highlighted how the pandemic has exacerbated the mental health crisis in youth .
The good news is that even small amounts of physical activity can immediately reduce symptoms of anxiety in adults and older adults. Depression has also shown to be responsive to physical activity. Research suggests that increased physical activity, of any kind, can improve depression symptoms experienced by people across the lifespan. Engaging in regular physical activity has also been shown to reduce the risk of developing depression in children and adults.
Though the seasons and our life circumstances may change, our basic needs do not. Just as we shift from shorts to coats or fresh summer fruits and vegetables to heartier fall food choices, so too must we shift our seasonal approach to how we stay physically active. Some of that is simply adapting to conditions: bundling up for a walk, wearing the appropriate shoes, or playing in the snow with the kids instead of playing soccer in the grass.
Sometimes there’s a bit more creativity involved. Often this means finding ways to simplify activity or make it more accessible. For example, it may not be possible to get to the gym or even take a walk due to weather or any number of reasons. In those instances, other options include adding new types of movement — such as impromptu dance parties at home — or doing a few household chores (yes, it all counts as physical activity).
During the COVID-19 pandemic, I built a makeshift gym in my garage as an alternative to driving back and forth to the gym several miles from home. That has not only saved me time and money but also afforded me the opportunity to get 15 to 45 minutes of muscle-strengthening physical activity in at odd times of the day.
For more ideas on how to get active — on any day — or for help finding the motivation to get started, check out this Move Your Way® video .
The point to remember is that no matter the approach, the Physical Activity Guidelines recommend that adults get at least 150 minutes of moderate-intensity aerobic activity (anything that gets your heart beating faster) each week and at least 2 days per week of muscle-strengthening activity (anything that makes your muscles work harder than usual). Youth need 60 minutes or more of physical activity each day. Preschool-aged children ages 3 to 5 years need to be active throughout the day — with adult caregivers encouraging active play — to enhance growth and development. Striving toward these goals and then continuing to get physical activity, in some shape or form, contributes to better health outcomes both immediately and over the long term.
For youth, sports offer additional avenues to more physical activity and improved mental health. Youth who participate in sports may enjoy psychosocial health benefits beyond the benefits they gain from other forms of leisure-time physical activity. Psychological health benefits include higher levels of perceived competence, confidence, and self-esteem — not to mention the benefits of team building, leadership, and resilience, which are important skills to apply on the field and throughout life. Research has also shown that youth sports participants have a reduced risk of suicide and suicidal thoughts and tendencies. Additionally, team sports participation during adolescence may lead to better mental health outcomes in adulthood (e.g., less anxiety and depression) for people exposed to adverse childhood experiences. In addition to the physical and mental health benefits, sports can be just plain fun.
Physical activity’s implications for significant positive effects on mental health and social well-being are enormous, impacting every facet of life. In fact, because of this national imperative, the presidential executive order that re-established the President’s Council on Sports, Fitness & Nutrition explicitly seeks to “expand national awareness of the importance of mental health as it pertains to physical fitness and nutrition.” While physical activity is not a substitute for mental health treatment when needed and it’s not the answer to certain mental health challenges, it does play a significant role in our emotional and cognitive well-being.
No matter how we choose to be active during the holiday season — or any season — every effort to move counts toward achieving recommended physical activity goals and will have positive impacts on both the mind and the body. Along with preventing diabetes, high blood pressure, obesity, and the additional risks associated with these comorbidities, physical activity’s positive effect on mental health is yet another important reason to be active and Move Your Way .
As for me… I think it’s time for a walk. Happy and healthy holidays, everyone!
Yours in health, Paul
Paul Reed, MD Rear Admiral, U.S. Public Health Service Deputy Assistant Secretary for Health Director, Office of Disease Prevention and Health Promotion

The Office of Disease Prevention and Health Promotion (ODPHP) cannot attest to the accuracy of a non-federal website.
Linking to a non-federal website does not constitute an endorsement by ODPHP or any of its employees of the sponsors or the information and products presented on the website.
You will be subject to the destination website's privacy policy when you follow the link.
- Search Menu
- Sign in through your institution
- Supplements
- Cohort Profiles
- Education Corner
- Author Guidelines
- Submission Site
- Open Access
- About the International Journal of Epidemiology
- About the International Epidemiological Association
- Editorial Team
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the IEA
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Cardiovascular and associated metabolic disease.
- < Previous
Physical activity and health: current issues and research needs
- Article contents
- Figures & tables
- Supplementary Data
Adrianne E Hardman, Physical activity and health: current issues and research needs, International Journal of Epidemiology , Volume 30, Issue 5, October 2001, Pages 1193–1197, https://doi.org/10.1093/ije/30.5.1193
- Permissions Icon Permissions
A substantial body of evidence now demonstrates the burden of ill-health attributable to sedentary living. This is most compelling for coronary heart disease (CHD) and, combined with the high prevalence of inactivity, 1 provides the rationale for Professor Morris's claim that exercise is 'today's best buy in public health'. 2 Besides a reduced risk of CHD, evidence is secure for many other health gains from physical activity; these include a reduced risk of stroke, 3, 4 type II diabetes, 5, 6 colon cancer, 7, 8 and hip fracture. 9, 10 There is evidence enough to justify the further development of public health policies to promote physical activity. The difficulty is with the specifics of what to promote and prescribe.
This paper is concerned with future contributions by research to an evidence-based rationale for exercise recommendations—both to the public at large and to individuals. It is clear that physically active people have a lower disease risk than sedentary individuals but the components of activity which determine particular health gains are poorly understood. Thus the 'dose-response' relationships for physical activity are the subject of current research interest. Intuitively, these will not be the same for different health outcomes and this is one reason why further study of the associated mechanisms is important. Understanding the underlying mechanisms will clarify the relative importance of intensity, frequency, duration and mode of exercise for specified health gains. It will also help us to distinguish the effects of exercise per se from those of co-existing behaviours and to identify stages of life during which levels of particular types of activity are critical for given health outcomes. This paper presents a personal view of research needs.
How important is intensity?
The rate of energy expenditure (in oxygen uptake units) of common physical activities is expressed in METS. One MET is equivalent to the resting metabolic rate, assumed to be 3.5 ml oxygen per kg of body mass per minute.
Oxygen uptake reserve is obtained by subtracting one MET (3.5 ml . kg .–1 min –1 ) from the maximal oxygen uptake.
Its importance in the epidemiology of physical activity is evidenced by data from British civil servants. 12 Whereas only frequent vigorous exercise (defined as liable to entail peaks of energy expenditure of ≥7.5 kcal.min –1 [31.5 kJ.min –1 ]) was associated with protection against heart attack in men aged 45–54 at entry, there was a dose-response relationship for a lesser degree of such exercise (either <2 sessions per week or not so intense, e.g. 'fairly brisk' walking for >30 min. per day) among older men aged 55–64 at entry. Thus, for example, older men reporting moderately intense activity such as 'much stair climbing' (not judged sufficiently vigorous to be included in the 'vigorous aerobic' cluster of activities) showed a coronary rate which was significantly lower than that in less active men. Protection among younger men was limited to those reporting frequent vigorous aerobic exercise. This finding suggests that the key features of cardio-protective exercise include its intensity relative to individual capacity. V • O 2 max declines, on average, by about 10% per decade in middle-aged and older people, 13 so exercise of a given MET value represents a higher relative intensity for older people. Where the number of individuals surveyed permit, one approach 14 may be to express the MET value of the activity in relation to age-related average values for oxygen uptake reserve.
Frequency of exercise
Recent recommendations 15, 16 are for exercise on '… most, preferably all, days of the week', underlining the importance of frequent exercise. This notion reflects increasing recognition of the acute effects of exercise, i.e. altered physiological or metabolic responses lasting between several hours and a few days after a session of exercise. These include a decrease in blood pressure, 17 improved insulin sensitivity 18 and decreases in plasma triglycerides. 19 The time-courses over which they disappear are poorly understood, however. Some information is available, for example the attenuation of the postprandial rise in plasma triglycerides following a standard high-fat meal has been reported to disappear within 60 hours of an exercise session. 20 Improved insulin sensitivity may persist for a little longer. 21 More information is required, however, as the duration of these effects dictates the frequency with which exercise sessions must be taken if favourable postprandial responses are to be maintained. Similarly, the determinants of the magnitude of acute effects of exercise need to be elucidated. Theoretically, this may be enhanced by training 22 because training permits more frequent and longer exercise sessions to be accomplished without fatigue. To the author's knowledge, this proposition has seldom been tested. 23
Pattern of exercise
Epidemiological studies have found an inverse relationship between the total energy expended in leisure time physical activity and health outcomes. These include a lower risk of all-cause mortality, 24 cardiovascular morbidity and mortality, 24, 25 type II diabetes, 6 hypertension, 26 and site-specific cancers. 27, 28 Some activities contributing to high totals of energy expenditure seem likely to have been performed at least partly on an intermittent basis, for example walking, 29 climbing stairs, 25, 30 gardening, 29 and repair work. 24 Survey evidence therefore suggests that several short sessions of moderate physical activity during the day influence health outcomes in a positive manner, at least when they contribute to a high total energy expenditure.
Scientific evidence for the efficacy of this pattern of exercise as a means of eliciting chronic (training) effects is limited however, both in the number of randomly controlled trials (three to the author's knowledge) and scope (the only common outcome measure was fitness). 31 Evidence is limited to scientific studies with outcome measures primarily of fitness and/or fatness. Only one study reported the effect of exercise pattern on acute health-related responses. This found similar reductions in plasma triglycerides with three, 10-minute bouts of brisk walking at intervals during the day and one, 30-minute bout in sedentary people consuming normal meals. 32
Further research is clearly required before the principle of accumulating exercise in short bouts throughout the day can be endorsed with confidence.
Energy expenditure and energy turnover
The product of intensity, frequency and duration of exercise—sometimes described as the total 'volume' of exercise (a difficult term)—yields the total gross energy expenditure. Some evidence points to this as an important determinant of health gains. In addition to the surveys referred to above, this includes the finding from the US Runners' Health Study that running mileage was six times more important in predicting high density lipoprotein cholesterol concentration than running speed. 33 This was not the case for associations with blood pressure or waist circumference, however, where running speed was the more important determinant. 33 Total energy expenditure may also be the main determinant of some acute effects of exercise. Two examples are relevant. First, the increase in glucose disposal rate was similar following exercise at 50% or 75% V • O 2 max when the total energy expended was held constant. 34 Second, the attenuation of postprandial plasma triglycerides by prior exercise was strikingly similar following a long bout of low intensity exercise and a shorter bout of moderate exercise expending the same energy. 35 This topic, again under-researched, is related to that of the accumulation of exercise (referred to above) because that enshrines the notion that the total energy expenditure is all-important.
Of course, in free-living people, an increased level of physical activity is invariably associated with an increase in energy intake so that energy turnover is increased. Speculatively, a higher energy turnover may constitute a metabolically desirable state because of effects on the pathways concerned with the disposition, storage and degradation of muscle energy substrates. Evidence for the health gains from such a state include the finding that men who were classified as obese by body mass index (BMI) but who had a high level of physical fitness had lower cardiovascular and total mortality rates than lean men who were unfit. 36 Similarly, although both high BMI and a high energy intake were associated with increased risk of colon cancer among inactive people, this was not the case among physically active individuals. 8 This finding suggests that a high energy intake does not confer increased risk of this cancer in the presence of a high expenditure.
The suggestion that a high energy turnover is metabolically advantageous is not new. The term 'metabolic fitness' was introduced by Després and Lamarche, 37 on the basis of a series of studies showing that change to plasma lipoprotein lipids and body fatness were achieved through high-volume, low intensity training in the absence of increases in V • O 2 max. Efforts to test this hypothesis through comparing the effects of 'lifestyle' activity with those of traditional exercise programmes have recently been reported 38– 40 but information is needed for a variety of health outcomes in different populations.
Over the last decade, epidemiological data on physical activity (a behaviour) has been complemented by findings based on physical fitness (a set of attributes related to the ability to perform exercise). These studies show a dose-response relationship so that, although men in the highest fitness groups consistently show the lowest coronary attack and total mortality rates, moderate levels of fitness also confer a statistically significant and clinically important reduction in risk. 41, 42 Physical fitness, because it is probably a more objective measure than physical activity is an attractive outcome measure. Its use could be extended of course if it could be measured satisfactorily outside the laboratory. A low-cost, rapid, non-intimidating method for this would allow large surveys with the statistical power to detect, for example, effects in sub-groups and effects of specific activities. Walking tests such as the UKK Institute's 2 km protocol 43 are attractive for both practical and theoretical reasons. Performance on these tests measures not only functional capacity (V • O 2 max, the most frequently used laboratory measure), but also endurance. This is defined as the capability to sustain aerobic exercise using a high proportion of V • O 2 max. Endurance is more sensitive to changes in physical activity level than V • O 2 max and, because it derives largely from metabolic adaptations in muscle, may be a more important determinant of related health gains.
As mentioned, epidemiological studies show associations between fitness and a variety of health outcomes. The need to elucidate the relationships between the 'dose' and pattern of activity and the health outcome has been mentioned above. Fitness (particularly endurance) is labile and so rather easily changed through short-term interventions. It therefore offers a means of studying these dose-response relations indirectly (but inexpensively), serving a link between the behaviour and health outcomes.
Most epidemiological studies have classified physical activities according to estimated energy expenditure—either totals or threshold rates. Recommendations to the public (whether direct or via health professionals), however, need to promote activities rather than energy expenditures. Walking is an obvious example. It is popular, inexpensive and carries a low risk of injury. It is often the most commonly reported activity, particularly among women 44 and older men. 12 Some landmark studies, including those by Professor Morris's group, 12 have published separate analyses for walking. 25 In British civil servants brisk walking accounted for over half of the exercise which was protective against heart attack in 55–64-year-old men. 12 Protection from attack among fairly brisk walkers was not significantly affected by controlling for participation in sports and cycling or for a lot of other CHD predictive factors. In recent years more data has become available, however. In the US Nurses Health Study, for example, walking was inversely associated with coronary events; women in the highest quintile group for walking (≥3 h per week at a brisk pace) had a multivariate relative risk of 0.65 (95% CI : 0.47–0.91). 45 Similarly, healthy older men in the Honolulu Heart Study who walked >1.5 miles per day had half the coronary risk of those who walked <0.25 miles per day. 46 Walking has also been reported to be associated with a lower risk of type II diabetes 47 (independently of participation in vigorous activity).
These observations are consistent with reports that moderate levels of fitness, associated with a reduction in all-cause mortality, are attainable through brisk or fast walking. 48, 49 Bearing in mind that sedentary people seldom exert themselves at more than 30–35% of V • O 2 max, 50 such walking is sufficiently vigorous to improve fitness in a majority of people whose health is at risk because of their inactivity.
Walking is especially suitable for older people and the functional gains it elicits will likely improve quality of life. It is plainly acceptable for them, and carries a low risk of injury. In 13 weeks of training by walking, only one injury was sustained among 57 healthy men and women their 70s. 51 Among older people, regular walking has been associated with lower rates of hospitalization, 52 lower plasma triglycerides and higher bone mineral density. 53
Because it is accessible to all but the very frail, more information on the specific benefits from walking—according to pace and distance—is sorely needed.
Studies of the associations between physical activity habits and disease outcomes must be complemented by research into the underlying mechanisms. Not only does this increase confidence that such associations may be causal but it helps us to understand the relative importance of the different components of exercise as mediators of specified health gains. For cardiovascular disease much is known of the potential contribution from exercise-induced changes to blood lipids, with recent information about considerable effects on the dynamic postprandial phase. Other mechanisms must be involved, however, because patients with CHD get improved myocardial perfusion (and decreased risk of further episodes) without net regression. 54
Recent findings suggest effects on the acute phases of the disease. (This would be concordant with observations that only continuing, current exercise confers a lower risk; past exercise has no effect. 12, 55 ) These include improved flow-mediated dilatation. 56 There may be links here with lipoprotein metabolism because flow-mediated dilatation is impaired by high plasma triglycerides, in proportion to concentration. 57
Mechanisms need elucidating in other areas, for instance skeletal health. Is the lower risk of hip fracture among physically active older women due to a decreased risk of falling and/or to an effect on bone mineral density? Is physical activity level particularly important during the years when bone formation predominates? The relationship between physical activity and a reduced risk of colon cancer is among the most consistent finding in the epidemiological literature. Is the mechanism systemic (reduced growth-promoting milieu) or local (increased colonic peristalsis)? Women who regularly engage in exercise may have a lower risk of breast cancer. 58 Speculation on potential mechanisms has involved endocrine factors and/or improved weight maintenance. Depending on the answers to such questions, some forms and regimens of exercise may be more effective than others in the achievement of particular objectives.
Physical inactivity is a waste of human potential for health and well-being and its high prevalence is a cause for concern. Its potential contribution to positive health (not merely the absence of disease but associated with a capacity to enjoy life and to withstand challenges 16 ) is considerable. So much is known—yet we need to understand much more. The effective 'dose' of exercise needed to elicit effects likely to be of clinical importance must be defined and this information translated into practical advice readily understood by the population at risk. Ten years after Professor Morris's plea for 'physiology and epidemiology to get together', 12 the need for co-operative efforts from these disciplines is even more urgent.
'Thank you'
I thank Professor Morris for posing thought-provoking questions and for stimulating discussion of these. His contributions—to research, to the National Fitness Survey for England, and to the development of public health policies—are valued by so many. It continues to be an education and a privilege to work with him.
National Fitness Survey: Main Findings. London: Sports Council and Health Education Authority, 1992.
Morris JN. Exercise in the prevention of coronary heart disease: today's best buy in public health. Med Sci Sports Exerc 1994 ; 26 : 807 –14.
Wannamethee G, Shaper AG. Physical activity and stroke in British middle-aged men. Br Med J 1992 ; 304 : 597 –601.
Lee I-M, Paffenbarger RS. Physical activity and stroke incidence. The Harvard Alumni Health Study. Stroke 1998 ; 29 : 2049 –54.
Haapanen N, Miilunpalo S, Vuori I, Oja P, Pasanen M. Association of leisure time physical activity with the risk of coronary heart disease, hypertension and diabetes in middle-aged men and women. Int J Epidemiol 1997 ; 26 : 739 –47.
Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991 ; 325 : 147 –52.
Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Int Med 1995 ; 122 : 327 –34.
Slattery ML, Potter J, Caan B et al . Energy balance and colon cancer—beyond physical activity. Cancer Res 1997 ; 57 : 75 –80.
Cummings SR, Nevitt MC, Browner WS et al . Risk factors for hip fracture in white women. N Engl J Med 1995 ; 332 : 767 –73.
Dargen-Molina P, Favier F, Grandjean H et al . Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet 1996 ; 348 : 145 –49.
Swain DP, Leutholtz BC. Heart rate reserve is equivalent to %V • O 2 Reserve, not to %V • O 2max . Med Sci Sports Exerc 1997 ; 29 : 410 –14.
Morris JN, Clayton DG, Everitt MG, Semmence AM, Burgess EH. Exercise in leisure time: coronary attack and death rates. Br Heart J 1990 ; 63 : 325 –34.
Hagberg JM. Effect of training on the decline of V • O 2max with aging. Fed Proc 1987 ; 46 : 1830 –33.
Howley ET. Type of activity: resistance, aerobic, anaerobic and leisure-time versus occupational physical activity. Med Sci Sports Exerc 2001 (in press).
More People, More Active, More Often . Report of Physical Activity Task Force. London: Department of Health, 1995.
Physical Activity and Health. A Report of the Surgeon General . US Departments of Health and Human Services. Centers for Disease Control and Prevention. Atlanta, GA, 1996.
Pescatello LS, Fargo AE, Leach CN, Scherzer HH. Short-term effect of dynamic exercsie on arterial blood pressure. Circulation 1991 ; 83 : 1557 –61.
Dela F, Mikines KJ, von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol 1992 ; 263 : E1134 –43.
Tsetsonis NV, Hardman AE, Mastana SS. Acute effects of exercise on postprandial lipemia: a comparative study in trained and untrained middle-aged women. Am J Clin Nutr 1997 ; 65 : 525 –33.
Hardman AE, Lawrence JEM, Herd SL. Postprandial lipemia in endurance-trained people during a short interruption to training. J Appl Physiol 1998 ; 84 : 1895 –901.
King DS, Baldus RJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J Appl Physiol 1995 ; 78 : 17 –22.
Haskell WL. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports Exerc 1994 ; 26 : 649 –60.
Crouse SF, O'Brien BC, Grandjean PW, Lowe RC, Rohack JJ, Green JS. Effects of training and a single session of exercise on lipids and apolipoproteins in hypercholesterolemic men. J Appl Physiol 1997 ; 83 : 2019 –28.
Haapanen N, Miilunpalo S, Vuori I, Oja P, Pasanen M. Characteristics of leisure time physical activity associated with decreased risk of premature all-cause and cardiovascular disease mortality in middle-aged men. Am J Epidemiol 1996 ; 143 : 870 –80.
Paffenbarger RS, Hyde RT, Wing AL, Hsieh C-C. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986 ; 314 : 605 –13.
Paffenbarger RS, Wing AL, Hyde RT, Jung DL. Physical activity and incidence of hypertension in college alumni. Am J Epidemiol 1983 ; 117 : 247 –57.
Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer 1996 ; 73 : 1134 –40.
Thune I, Brenn T, Lund E, Gaard M. Physical activity and the risk of breast cancer. N Engl J Med 1997 ; 336 : 1269 –75.
Magnus K, Matroos A, Strackee J. Walking, cycling, or gardening, with or without seasonal interruption, in relation to coronary events. Am J Epidemiol 1979 ; 110 : 724 –33.
Paffenbarger RS, Hyde RT, Wing AL, Lee I-M, Jung DL, Kampert JB. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993 ; 328 : 538 –45.
Hardman AE. Issues of fractionalization (intermittent versus continuous exercise). Med Sci Sports Exerc 2001 ; 33 (6Suppl.): S421 –27.
Murphy MH, Nevill AM, Hardman AE. Different patterns of brisk walking are equally effective in decreasing postprandial lipaemia. Int J Obesity 2000 ; 24 : 1303 –09.
Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch Int Med 1998 ; 158 : 237 –45.
Braun B, Zimmermann MB, Kretchmer N. Effects of exercise intensity on insulin sensitivity in women with non-insulin-dependent diabetes mellitus. J Appl Physiol 1995 ; 78 : 300 –06.
Tsetsonis NV, Hardman AE. Reduction in postprandial lipemia after walking: influence of exercise intensity. Med Sci Sports Exerc 1996 ; 28 : 1235 –42.
Wei M, Kampert JB, Barlow CE et al . Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. J Am Med Assoc 1999 ; 282 : 1547 –53.
Després J-P, Lamarche B. Low-intensity endurance exercise training, plasma lipoproteins and the risk of coronary heart disease. J Int Med 1994 ; 236 : 7 –22.
Andersen RE. Effects of lifestyle activity vs structured aerobic exercise in obese women. A randomized trial. J Am Med Assoc 1999 ; 281 : 335 –40.
Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women. A randomized trial. J Am Med Assoc 1999 ; 282 : 1554 –60.
Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness. J Am Med Assoc 1999 ; 281 : 327 –34.
Lakka TA, Venäläinen JM, Raurama R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction in men. N Engl J Med 1994 ; 330 : 1549 –54.
Blair SN, Kampert JB, Kohl HW et al . Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. J Am Med Assoc 1996 ; 276 : 205 –10.
Oja P, Laukkanen R, Pasanen M, Tyry T, Vuori I. A 2-km walking test for assessing cardiorespiratory fitness in healthy adults. Int J Sports Med 1991 ; 12 : 356 –62.
Lemaitre RN, Heckbert SR, Psaty BM, Siscovick DS. Leisure-time physical activity and the risk of nonfatal myocardial infarction in postmenopausal women. Arch Int Med 1995 ; 155 : 2302 –08.
Manson J, Hu FB, Rich-Edwards JW et al . A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999 ; 341 : 650 –58.
Hakim AA, Curb JD, Petrovitch H et al . Effects of walking on coronary heart disease in elderly men. The Honolulu Heart Program. Circulation 1999 ; 100 : 9 –13.
Hu FB, Sigal RJ, Rich-Edwards JW et al . Walking compared with vigorous physical activity and risk of type 2 diabetes in women. J Am Med Assoc 1999 ; 282 : 1433 –39.
Stensel DJ, Brooke-Wavell K, Hardman AE, Jones PRM, Norgan NG. The influence of a one year programme of brisk walking on endurance fitness and body composition in previously sedentary men aged 42–59 years. Eur J Appl Physiol 1994 ; 68 : 531 –37.
Hardman AE, Jones PRM, Norgan NG, Hudson A. Brisk walking improves endurance fitness without changing body fatness in previously sedentary women. Eur J Appl Physiol 1992 ; 65 : 354 –59.
Franklin BA, Blair SN, Haskell WL, Thompson PD, Van Camp SP. Exercise and cardiac complications. Do the benefits outweigh the risks? Physician Sportsmed 1994 ; 22 : 56 –68.
Pollock ML, Carroll JF, Graves JE et al . Injuries and adherence to walk/jog and resistance training programs in the elderly. Med Sci Sports Exerc 1991 ; 23 : 1194 –200.
LaCroiz AZ, Leveille SG, Hecht JA, Grothaus LC, Wagner EH. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults? J Am Geriatr Soc 1996 ; 44 : 113 –20.
Frändin K, Grimby G, Mellström D, Svanborg D. Walking habits and health-related factors in a 70-year-old population. Gerontology 1991 ; 37 : 281 –88.
Schuler G, Hambrecht R, Schlierf G et al . Regular physical exercise and low-fat diet: effects on the progression of coronary artery disease. Circulation 1992 ; 86 : 1 –11.
Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 1978 ; 108 : 161 –75.
Hambrecht R, Wolf A, Gielen S et al . Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 2000 ; 342 : 454 –60.
Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997 ; 79 : 350 –54.
Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst 1994 ; 86 : 1403 –08.
| Month: | Total Views: |
|---|---|
| January 2017 | 35 |
| February 2017 | 135 |
| March 2017 | 65 |
| April 2017 | 27 |
| May 2017 | 55 |
| June 2017 | 19 |
| July 2017 | 25 |
| August 2017 | 83 |
| September 2017 | 83 |
| October 2017 | 98 |
| November 2017 | 167 |
| December 2017 | 488 |
| January 2018 | 730 |
| February 2018 | 770 |
| March 2018 | 1,177 |
| April 2018 | 1,134 |
| May 2018 | 1,399 |
| June 2018 | 1,095 |
| July 2018 | 981 |
| August 2018 | 1,362 |
| September 2018 | 1,670 |
| October 2018 | 1,772 |
| November 2018 | 1,660 |
| December 2018 | 1,179 |
| January 2019 | 908 |
| February 2019 | 1,097 |
| March 2019 | 1,123 |
| April 2019 | 1,063 |
| May 2019 | 1,006 |
| June 2019 | 1,101 |
| July 2019 | 1,505 |
| August 2019 | 1,390 |
| September 2019 | 1,707 |
| October 2019 | 1,141 |
| November 2019 | 931 |
| December 2019 | 711 |
| January 2020 | 829 |
| February 2020 | 721 |
| March 2020 | 724 |
| April 2020 | 649 |
| May 2020 | 323 |
| June 2020 | 399 |
| July 2020 | 467 |
| August 2020 | 724 |
| September 2020 | 1,066 |
| October 2020 | 1,294 |
| November 2020 | 1,282 |
| December 2020 | 885 |
| January 2021 | 1,151 |
| February 2021 | 1,420 |
| March 2021 | 1,713 |
| April 2021 | 971 |
| May 2021 | 645 |
| June 2021 | 496 |
| July 2021 | 405 |
| August 2021 | 808 |
| September 2021 | 601 |
| October 2021 | 565 |
| November 2021 | 684 |
| December 2021 | 382 |
| January 2022 | 473 |
| February 2022 | 408 |
| March 2022 | 577 |
| April 2022 | 436 |
| May 2022 | 452 |
| June 2022 | 279 |
| July 2022 | 189 |
| August 2022 | 199 |
| September 2022 | 237 |
| October 2022 | 231 |
| November 2022 | 205 |
| December 2022 | 164 |
| January 2023 | 138 |
| February 2023 | 141 |
| March 2023 | 179 |
| April 2023 | 130 |
| May 2023 | 128 |
| June 2023 | 95 |
| July 2023 | 78 |
| August 2023 | 88 |
| September 2023 | 57 |
| October 2023 | 82 |
| November 2023 | 95 |
| December 2023 | 75 |
| January 2024 | 87 |
| February 2024 | 91 |
| March 2024 | 90 |
| April 2024 | 149 |
| May 2024 | 107 |
| June 2024 | 39 |
Email alerts
Citing articles via, looking for your next opportunity.
- About International Journal of Epidemiology
- Recommend to your Library
Affiliations
- Online ISSN 1464-3685
- Copyright © 2024 International Epidemiological Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Health Education
- Health Education and Promotion
- Public Health
- Physical Fitness
Physical Fitness, Exercise Self-Efficacy, and Quality of Life in Adulthood: A Systematic Review
- August 2020
- 17(17):6343
- This person is not on ResearchGate, or hasn't claimed this research yet.

- University of Cordoba (Spain)

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Eleonor I. Fransson

- Agustin Mora-Fernandez
- Josue González-Ruiz
- Miguel Mariscal-Arcas
- Awan Hariono Hariono
- Budi Aryanto
- Herwin Herwin
- Agung Nugroho

- Naiche Chen

- Saed Nikookheslat
- Fariba Arbabi
- Siamak Rahbar

- Heikki Kyröläinen

- IND MANAGE DATA SYST
- Vincent C.S. Lee
- Haiyan Wang

- Timothy E. McAlindon

- Danilo Yuzo Nishimoto
- Giovana Damasceno e Souza

- INT J BEHAV NUTR PHY

- J Neurol Phys Ther

- Ignacio Moreno-Suarez
- Sylvia Liew
- Lawrence G. Dembo

- Steven N Blair
- In Yae Cheong

- Sharon L. Brennan-Olsen

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
REVIEW article
Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits.

- 1 Department of Movement Sciences and Wellbeing, Parthenope University of Naples, Naples, Italy
- 2 IRCCS Fondazione Santa Lucia, Rome, Italy
- 3 Istituto di Diagnosi e Cura Hermitage Capodimonte, Naples, Italy
- 4 Department of Medical and Surgical Sciences, Magna Graecia University, Catanzaro, Italy
- 5 Department of Science and Technology, Parthenope University of Naples, Naples, Italy
- 6 Department of Engineering, Parthenope University of Naples, Naples, Italy
- 7 Institute of Applied Sciences and Intelligent Systems, CNR, Pozzuoli, Italy
Much evidence shows that physical exercise (PE) is a strong gene modulator that induces structural and functional changes in the brain, determining enormous benefit on both cognitive functioning and wellbeing. PE is also a protective factor for neurodegeneration. However, it is unclear if such protection is granted through modifications to the biological mechanisms underlying neurodegeneration or through better compensation against attacks. This concise review addresses the biological and psychological positive effects of PE describing the results obtained on brain plasticity and epigenetic mechanisms in animal and human studies, in order to clarify how to maximize the positive effects of PE while avoiding negative consequences, as in the case of exercise addiction.
Introduction
Many evidences demonstrated that physical exercise (PE) affects brain plasticity, influencing cognition and wellbeing ( Weinberg and Gould, 2015 ; for review see Fernandes et al., 2017 ). In fact, experimental and clinical studies have reported that PE induces structural and functional changes in the brain, determining enormous biological, and psychological benefits.
In general, when reported PE effects, it is customary to separate the biological aspects from the psychological ones. In fact, most of the studies documented either the effects of PE on the brain (and then on the cognitive functioning) or on the wellbeing (in terms of physical and mental health). In this review, we merge both these aspects as they influence each other. In fact, behaviorally appropriate choices depend upon efficient cognitive functioning. Furthermore, emotional states influence cognitive functions through specific cerebral circuitry involving prefrontal areas and limbic structures ( Barbas, 2000 ).
Before analyzing the benefits of PE, it is necessary to define PE precisely. Indeed, PE is a term often incorrectly used interchangeably with physical activity (PA) that is “any bodily movement produced by skeletal muscles that requires energy expenditure” ( World Health Organization, 2010 ). Then, PA includes any motor behavior such as daily and leisure activities and it is considered a determinant lifestyle for general health status ( Burkhalter and Hillman, 2011 ). Instead, PE is “a sub classification of PA that is planned, structured, repetitive, and has as a final or an intermediate objective the improvement or maintenance of one or more components of physical fitness” ( World Health Organization, 2010 ). Examples of PE are aerobic and anaerobic activity, characterized by a precise frequency, duration and intensity.
In this review, we illustrate the biological and psychological benefits of PE on cognition and wellbeing both in health and diseases, reporting data from both animal and human studies. The biological basis at both molecular and supramolecular level have been largely studied. The other aim of present work is to report the actual evidence on the epigenetic mechanisms that determine or modulate the biological effects of PE on the brain. In fact, while the biologic mechanisms are sufficiently studied both at the molecular and supramolecular levels (see Lista and Sorrentino, 2010 ), little is known about the epigenetic ones. Finally, the modality with which PE should be practiced to gain such advantages while avoiding negative consequences will be discussed. In Table 1 are reported the inclusion and exclusion criteria for studies discussed in this review.
Table 1 . Inclusion and exclusion criteria for studies included in this review.
Physical Exercise, Brain, and Cognition
Among the biological effects of PE, those linked to “neuroplasticity” are quite important.
Neuroplasticity is an important feature of the nervous system, which can modify itself in response to experience ( Bavelier and Neville, 2002 ). For this reason, PE may be considered as an enhancer environmental factor promoting neuroplasticity.
In animal studies, the structural changes analyzed concern the cellular (neurogenesis, gliogenesis, synaptogenesis, angiogenesis) and molecular (alteration in neurotransmission systems and increasing in some neurotrophic factors) level ( Gelfo et al., 2018 ), while the functional activity has been measured using the levels of performance in behavioral tasks, such as spatial tasks that allow to analyze the different facets of spatial cognitive functions ( Mandolesi et al., 2017 ). In humans, indicators of structural changes correspond for example to brain volumes, measures of white matter integrity or modulation in neurotrophins levels (by correlation with trophic factors plasma levels). Such metrics can be correlated to cognitive performances, defining the functional neural efficiency ( Serra et al., 2011 ). To this regard, it should be emphasized that any morphological change results in a modification of the functional properties of a neural circuit and vice versa any change in neuronal efficiency and functionality is based on morphological modifications ( Mandolesi et al., 2017 ).
Experimental and clinical studies have shown that PE induces important structural and functional changes in brain functioning. In Table 2 are reported the more evident effects induced by PE.
Table 2 . Structural and functional effects of PE.
Animal Studies
In animals, motor activity or motor exercise are terms often used instead of PE. The effects of motor exercise are mainly studied in rodents by means of specific training on wheels or by locomotor activity analyses.
Studies on healthy animals have demonstrated that intense motor activity increases neurons and glia cells proliferation rates in the hippocampus and the neocortex ( van Praag et al., 1999a , b ; Brown et al., 2003 ; Ehninger and Kempermann, 2003 ; Steiner et al., 2004 ; Hirase and Shinohara, 2014 ) and induces angiogenesis in the neocortex, hippocampus, and cerebellum ( Black et al., 1990 ; Isaacs et al., 1992 ; Kleim et al., 2002 ; Swain et al., 2003 ; Ekstrand et al., 2008 ; Gelfo et al., 2018 ). At the molecular level, motor activity causes changes in neurotrasmitters such as serotonin, noradrenalin, and acetylcholine ( Lista and Sorrentino, 2010 ; for a review, see Lin and Kuo, 2013 ) and induces the release of the brain-derived neurotrophic factor (BDNF Vaynman et al., 2004 ; Lafenetre et al., 2011 ) and the insulin-like growth factor-1 (IGF-1; for a review, van Praag, 2009 ).
Animals performing motor exercise showed improvements in spatial abilities ( van Praag et al., 2005 ; Snigdha et al., 2014 ) and in other cognitive domains such as executive functions ( Langdon and Corbett, 2012 ), evidencing thus that motor exercise improve cognitive functions.
Similar structural and functional changes were evident even in older animals ( Kronenberg et al., 2006 ) and in animal models of neurodegenerative diseases ( Nithianantharajah and Hannan, 2006 ), suggesting that motor exercise is a potent neuroprotective factor against physiological and pathological aging ( Gelfo et al., 2018 ). In this context, one can use transgenic models to determine exactly when a structural alteration occurs, and then to study when the animals should undergo motor training in order to maximize its effects. To this regard, converging evidence is showing that motor activity should be performed before the development of neurodegeneration in order to exert its protective role ( Richter et al., 2008 ; Lin et al., 2015 ) such as before the formation of beta amyloid plaques in Alzheimer's disease ( Adlard et al., 2005 ). However, there are some experimental evidences showing that motor exercise performed after neurodegenerative lesions permits to improve spatial abilities, hence being also a potent therapeutic agent ( Sim, 2014 ; Ji et al., 2015 ).
Interestingly, PE induces modifications that can be passed on to the offspring. In fact, positive maternal experiences can influence the offspring at both behavioral and biochemical levels (see Cutuli et al., 2017 , 2018 ). Preclinical studies also indicated that the effects of maternal exercise during pregnancy can be passed on to offspring ( Robinson et al., 2012 ). However, it is not clear if the possibilities of inheritance are limited to motor exercise alone. To this regard, it has been seen that pregnant rats exposed to motor exercise on wheel-running and treadmill running have offspring with improved spatial memory, and increased hippocampal BDNF level ( Akhavan et al., 2008 ; Aksu et al., 2012 ). However, further studies are necessary since it remains unclear whether these beneficial effects result from physiological changes to the in utero environment and/or from epigenetic modifications to the developing embryo ( Short et al., 2017 ). On the other hand, few studies, conflicting and hard to replicate, do not yet allow to explore the transgenerational effects of paternal motor exercise ( Short et al., 2017 ).
Human Studies
Neuroplasticity phenomena following PE have been evidenced even in humans. A great number of studies demonstrated that in adults, PE determines structural changes such as increased gray matter volume in frontal and hippocampal regions ( Colcombe et al., 2006 ; Erickson et al., 2011 ) and reduced damage in the gray matter ( Chaddock-Heyman et al., 2014 ).
Moreover, PE facilitates the release of neurotrophic factors such as peripheral BDNF ( Hötting et al., 2016 ), increases blood flow, improves cerebrovascular health and determines benefits on glucose and lipid metabolism carrying “food” to the brain ( Mandolesi et al., 2017 ).
These effects are reflected on cognitive functioning (for a review see Hötting and Röder, 2013 ). In fact, the results of cross-sectional and epidemiological studies showed that PE enhances cognitive functions in young and older adults ( Lista and Sorrentino, 2010 ; Fernandes et al., 2017 ), improving memory abilities, efficiency of attentional processes and executive-control processes ( Kramer et al., 1999 ; Colcombe and Kramer, 2003 ; Grego et al., 2005 ; Pereira et al., 2007 ; Winter et al., 2007 ; Chieffi et al., 2017 ). Furthermore, structural changes following PE have been related to academic achievement in comparison to sedentary individuals ( Lees and Hopkins, 2013 ; Donnelly et al., 2016 ). In this line, it has been also showed that children who practice regular aerobic activity performed better on verbal, perceptual and arithmetic test in comparison to sedentary ones of same age ( Sibley and Etnier, 2003 ; Voss et al., 2011 ).
Numerous studies have demonstrated that PE prevents cognitive decline linked to aging ( Yaffe et al., 2009 ; Hötting and Röder, 2013 ; Niemann et al., 2014 ), reduces the risk of developing dementia ( Colberg et al., 2008 ; Mandolesi et al., 2017 ), the level of deterioration in executive functions ( Hollamby et al., 2017 ) and improves the quality of life ( Pedrinolla et al., 2017 ). Furthermore, positron emission tomography based studies evidenced that PE determines changes in metabolic networks that are related to cognition ( Huang et al., 2016 ).
Recently, studies on magnetoencephalography based (MEG) functional connectivity evidenced that PE influences network topology ( Foster, 2015 ). It is important to underlie that MEG is a much more direct measure of neural activity in comparison to fRMI, with the advantage of combining good spatial and high temporal resolution. In healthy individuals, PE was related to better intermodular integration ( Douw et al., 2014 ) and to improvements in cognitive functions ( Huang et al., 2016 ). Benefits of PE are evidenced even in individuals at risk for AD ( Deeny et al., 2008 ), thus once again suggesting a protective role of PE.
A possible explanation for these ameliorative structural and functional effects could be that PE stimulates blood circulation in the neural circuits involved in cognitive functioning ( Erickson et al., 2012 ). Another interpretation could be found in the concept of “cerebral reserves” ( Stern, 2002 , 2012 ) a mechanisms that might explain why, in the face of neurodegenerative changes that are similar in nature and extent, individuals vary considerably in the severity of cognitive aging and clinical dementia ( Petrosini et al., 2009 ). Two types of reserves are recognized: brain reserve and cognitive reserve. The former is based on the protective potential of anatomical features such as brain size, neuronal density and synaptic connectivity, the latter is based on the efficient connectivity among neural circuits ( Stern, 2002 ; Mandolesi et al., 2017 ).
According to the reserves hypothesis and taking into account the numerous evidences described above, we could advance that PE is an environmental factor that permits to gain reserves.
However, one must underline that if on the one hand PE improves the cognitive functioning, providing reserves to be spent in the case of a brain lesion, on the other hand the modifications of the clinical expression of neurodegeneration delays the diagnosis. It has been seen that patients with higher cognitive reserve take longer to manifest the symptoms of memory loss ( Zanetti et al., 2017 ). It has been hypothesized a neural compensation mechanism that permits to perform complex activities ( Stern, 2009 ). Obviously, these conclusions open important reflections more for the diagnosis of neurodegenerative disease than for the practice of PE.
The effects of PE on cognitive functioning have been shown across the lifespan from childhood to the old age ( Hötting and Röder, 2013 ). In particular, it has been evidenced that cognitive functions that are influenced the most by brain maturation, such as attention or cognitive flexibility, and the cognitive functions that depend the most upon experiences, such as memory, are the most sensitive ones to PE ( Hötting and Röder, 2013 ). Overall, these studies, together with those analyzing the effects of combined environmental factors, suggest that for a positive effect on cognitive function, it is necessary to maintain an “enriched lifestyle” up to middle life. In fact, the exposure to PE together to other many experiences provides a “reserve”-like advantage which supports an enduring preservation of cognitive function in old age ( Chang et al., 2010 ; Loprinzi et al., 2018 ).
Physical Exercise and Wellbeing
There are consistent evidences that PE has many benefits for people of any age, improving psychological wellbeing ( Zubala et al., 2017 ) and quality of life ( Penedo and Dahn, 2005 ; Windle et al., 2010 ; Table 3 ).
Table 3 . Biological and psychological effects of PE (Adapted from Weinberg and Gould, 2015 ).
In children, PE is correlated with high levels of self-efficacy, tasks goal orientation, and perceived competence ( Biddle et al., 2011 ). In youth and adulthood, most studies evidenced that PE is associated with better health outcomes, such as better mood and self-concept ( Berger and Motl, 2001 ; Landers and Arent, 2001 ; Penedo and Dahn, 2005 ). In the aging population, PE helps maintaining independence ( Stessman et al., 2009 ), favoring social relations and mental health.
It was now well-accepted that is the interaction between biological and psychological mechanisms linked to PE enhances the wellbeing ( Penedo and Dahn, 2005 ). Biological mechanisms of beneficial effects of PE are mainly related to increasing in cerebral blood flow and in maximal oxygen consumption, to delivery of oxygen to cerebral tissue, to reduction in muscle tension and to increased serum concentrations of endocannabinoid receptors ( Thomas et al., 1989 ; Dietrich and McDaniel, 2004 ; Querido and Sheel, 2007 ; Gomes da Silva et al., 2010 ; Ferreira-Vieira et al., 2014 ). Moreover, neuroplasticity phenomena such as changes in neurotransmitters are recognized to affect wellbeing. For example, PE increases the levels of serotonin ( Young, 2007 ; Korb et al., 2010 ) and the levels of beta-endorphins, such as anandamide ( Fuss et al., 2015 ).
Among the psychological hypothesis proposed to explain how PE enhances wellbeing, it has been underlined feeling of control ( Weinberg and Gould, 2015 ), competency and self-efficacy ( Craft, 2005 ; Rodgers et al., 2014 ), improved self-concept and self-esteem ( Marsh and Sonstroem, 1995 ; Fox, 2000 ; Zamani Sani et al., 2016 ), positive social interactions and opportunities for fun and enjoyment ( Raedeke, 2007 ; Bartlett et al., 2011 ).
Psychological research evidenced that PE can even modulate the personality and the development of Self ( Weinberg and Gould, 2015 ). Moreover, PE has been correlated with hardiness, a personality style that enables a person to withstand or cope with stressful situations ( Weinberg and Gould, 2015 ).
In the following sections, we will focus on correlations among PE and the most common mental illnesses.
Depression and Anxiety
Depression is the most common type of mental illness and will be the second leading cause of disease by 2020 ( Farioli-Vecchioli et al., 2018 ). Similar entity concerns anxiety disorders that are among the most prevalent mental disorders in the world population ( Weinberg and Gould, 2015 ).
Epidemiological studies have consistently reported benefits of PE on reductions in depression ( Mammen and Faulkner, 2013 ) and anxiety ( DeBoer et al., 2012 ). For example, it has been seen that individuals that practice PE regularly are less depressed or anxious than those who do not ( De Moor et al., 2006 ), suggesting the use of exercise as a treatment for these illnesses ( Carek et al., 2011 ).
Most of the research on the relationship between PE and positive changes in mood state has evidenced positive effects, especially as a consequence of aerobic exercise, regardless of the specific type of activity ( Knapen et al., 2009 ), even if the correct intensity of aerobic PE to control and reduce symptoms is debated ( de Souza Moura et al., 2015 ). For example, it has been revealed that after about 16 weeks of an aerobic exercise program, individuals with major depressive disorder (MDD), significantly reduced their depressive symptoms ( Craft and Perna, 2004 ). However, there are evidenced that documented that even anaerobic activity has positive effects on treatment of clinical depression ( Martinsen, 1990 ). For anxiety disorders, it has been evidenced that the positive effects of PE are visible even with short bursts of exercise, independently from the nature of the exercise ( Scully et al., 1998 ).
A physiologic mechanism correlated to the improvement in depressed mood post-exercise PE was identified in modulation of peripheral levels of BDNF ( Coelho et al., 2013 ). In this line, it was suggested recently that the intensity of exercise to improve mood should be prescribed on individual basis and not on the patient's preferred intensity ( Meyer et al., 2016a , b ). Conversely, physical inactivity correlated to worse depressive symptoms and, then, to lower peripheral levels of BDNF ( Brunoni et al., 2008 ). Post-PE mood improvement might also be due to lower oxidative stress ( Thomson et al., 2015 ). In this contest, it was evidenced that there is an abnormal oxidative stress in individuals with MDD or bipolar disorder ( Cataldo et al., 2010 ; Andreazza et al., 2013 ) and that PE, particularly in higher intensity, decreases oxidative stress with consequent mood improvement ( Urso and Clarkson, 2003 ).
Addictive and Unhealthy Behaviors
PE has been widely evidenced to be an effective tool for treating several addictive and unhealthy behaviors. PE tends to reduce and prevent behaviors such as smoking, alcohol, and gambling, and to regulate the impulse for hunger and satiety ( Vatansever-Ozen et al., 2011 ; Tiryaki-Sonmez et al., 2015 ). In this context, several studies evidenced substance abusers benefit from regular PE, that also helps increasing healthy behaviors ( Giesen et al., 2015 ). It has been evidenced that regular PE reduces tobacco cravings and cigarette use ( Haasova et al., 2013 ). Although PE has positive effects on psychological wellbeing, in this context it is right underline that in some cases PE could reveal unhealthy behaviors with negative consequence on health ( Schwellnus et al., 2016 ). It is the case of exercise addiction, a dependence on a regular regimen of exercise that is characterized by withdrawal symptoms, after 24–36 h without exercise ( Sachs, 1981 ), such as anxiety, irritability, guilt, muscle twitching, a bloated feeling, and nervousness ( Weinberg and Gould, 2015 ). There is a strong correlation between exercise addiction and eating disorders ( Scully et al., 1998 ) suggesting thus a comorbidity of these disorders and a common biological substrate. In particular, recent studies have shown that these unhealthy behaviors are associated to lower prefrontal cortex volume, activity and oxygenation, with consequent impairment in cognitive functions, such as the inhibitory control with the consequent compulsive behaviors ( Asensio et al., 2016 ; Wang et al., 2016 ; Pahng et al., 2017 ). Also, it has been seen that a few days of PE increase oxygenation of prefrontal cortex, improving mental health ( Cabral et al., 2017 ).
Epigenetic Mechanisms
Biological and psychological effects of PE could be partly explained through epigenetic mechanisms. The term “epigenetics,” coined by Waddington (1939) , is based on a conceptual model designed to account for how genes might interact with their environment to produce the phenotype ( Waddington, 1939 ; Fernandes et al., 2017 ).
In particular, epigenetics is referred to all those mechanisms, including functional modifications of the genome such as DNA methylation, post-translational histone modifications (i.e., acetylation and methylation) and microRNA expression ( Deibel et al., 2015 ; Grazioli et al., 2017 ), which tend to regulate gene expression, modeling the chromatin structure but maintaining the nucleotide sequence of DNA unchanged.
The current literature clearly demonstrates that these mechanisms are strongly influenced by different biological and environmental factors, such as PE ( Grazioli et al., 2017 ), which determine the nature and the mode of epigenetic mechanisms activation.
Epigenetics plays an essential role in neural reorganization, including those that govern the brain plasticity ( Deibel et al., 2015 ). For example, a growing body of evidence indicates that regulates neuroplasticity and memory processes ( Ieraci et al., 2015 ).
Several animal studies reveal how motor activity is able to improve cognitive performances acting on epigenetic mechanisms and influencing the expression of those genes involved in neuroplasticity ( Fernandes et al., 2017 ). The main molecular processes that underlie the epigenetic mechanisms are the following: through DNA methylation, histone modifications and microRNA expression ( Fernandes et al., 2017 ).
DNA methylation is a chemical covalent modification on the cytosine of the double stranded DNA molecule. It has been recognized that DNA methylation plays a key role in long-term memory ( Deibel et al., 2015 ; Kim and Kaang, 2017 ). In particular, mechanisms related to DNA methylation relieve the repressive effects of memory-suppressor genes to favor the expression of plasticity-promoting and memory consolidation genes. Several evidences showed that PE is able to coordinate the action of the genes involved in synaptic plasticity that regulate memory consolidation ( Molteni et al., 2002 ; Ding et al., 2006 ).
Histone modifications are post-translational chemical changes in histone proteins. They include histone methylation/demethylation, acetylation/deacetylation, and phosphorylation, all due to the activity of specific enzymes, which modify the chromatin structure, thereby regulating gene expression. It has been demonstrated that histone acetylation is a requisite for long-term memory (LTM) ( Barrett and Wood, 2008 ; Fernandes et al., 2017 ). In animals, motor activity increases these genetic mechanisms in the hippocampus and the frontal cortex, improving memory performances in behavioral tasks ( Cechinel et al., 2016 ). Recently, following 4 weeks of motor exercise, it has been evidenced an increasing of the activity of enzymes involved in histone acetylation/deacetylation, the epigenetic mechanisms that determine an enhancing in the expression of BDNF ( Maejima et al., 2018 ).
MicroRNAs (miRNAs) are small, single stranded RNA molecules able to inhibit the expression of target genes. They are widely expressed in the brain, participating in epigenetic mechanisms and acting as regulators of numerous biological processes in the brain, ranging from cell proliferation, differentiation, apoptosis, synaptic plasticity, and memory consolidation ( Saab and Mansuy, 2014 ). Recent evidences demonstrate that PE can mitigate the harmful effects of traumatic brain injury and aging on cognitive function by regulating the hippocampal expression of miR21 ( Hu et al., 2015 ) and miR-34a ( Kou et al., 2017 ). Furthermore, PE contributes to attenuate the effects of stress-related increase in miR-124, involved in neurogenesis and memory formation ( Pan-Vazquez et al., 2015 ).
What Kind of Physical Exercise?
Sport psychology has suggested that the success or failure of PE programs depends on several factors such as the intensity, frequency, duration of the exercise, and whether the PE is done in group or alone ( Weinberg and Gould, 2015 ). These aspects are important in terms of maintenance of PE practice and in order to gain benefits for brain and behavior, and they are affected by individual characteristics. Although such aspects have to be taken into account when training is proposed, scientific reports have evidenced different effects on cognitive functioning and wellbeing if PE is performed in aerobic or anaerobic modality.
Aerobic exercise allows the resynthesis of adenosine—triphosphate (ATP) by aerobic mechanisms, adjusting intensity (from low to high intensity), duration (usually long), and oxygen availability. The intensity depends on the cardiorespiratory effort with respect to the maximum heart rate (HRmax) or the maximum oxygen consumption (Vo2max), which determines an increase in oxygen consumption with respect to the rest condition. Examples of aerobic PE are jogging, running, cycling, and swimming.
On the contrary, anaerobic exercise has high intensity, short duration and unavailability of oxygen, determining the depletion of the muscles' ATP and/or phosphocreatine (PCr) reserves, shifting the production of ATP, to anaerobic energy mechanisms, lactacid or alactacid. Examples of anaerobic exercises are weight lifting or sprint in 100 m.
Robust literature demonstrated that chronic aerobic exercise is associated with potent structural and functional neuroplastic changes, with an improvement in cognitive functions ( Colcombe et al., 2006 ; Hillman et al., 2008 ; Erickson et al., 2009 ; Mandolesi et al., 2017 ) and increased feeling of general wellbeing ( Berger and Tobar, 2011 ; Biddle et al., 2011 ) (Table 4 ).
Table 4 . Effects of physical aerobic exercise on cognitive functioning and wellbeing.
Recently, growing evidence showed that acute aerobic exercise, defined as a single bout of exercise, relates to improved cognitive functions, especially prefrontal cortex-dependent cognition ( Tomporowski, 2003 ; Lambourne and Tomporowski, 2010 ; Chang et al., 2011 ; Ludyga et al., 2016 ; Basso and Suzuki, 2017 ). However, the effects of a single session of exercise on cognitive functioning are generally small ( Chang et al., 2012 ). In this line, it was evidenced that even a single bout of moderate-intensity aerobic exercise enhances, mood and emotional states and improves the wellbeing in MDD individuals ( Bartholomew et al., 2005 ; Basso and Suzuki, 2017 ) (Table 4 ).
Beside frequency and duration over time, even the intensity is a parameter to be considered when evaluating the PE effects. It has been showed that moderate intensity exercise is related to increased performance in working memory and cognitive flexibility, whereas high-intensity exercise improves the speed of information processing ( Chang and Etnier, 2009 ). In this context, it has been reported that peripheral BDNF was significantly increased after high intensity exercise, but not after low-intensity exercise ( Hötting et al., 2016 ). In fact, it is evidenced that high-intensity exercise provides greater benefit to cognitive functions than low-intensity exercise in the elderly ( Brown et al., 2012 ).
With regard to the psychological beneficial effects related to PE, research has evidenced that major benefits in reduction of anxiety and depression are determined by longer training program (several months), as compared to shorter ones (some days) for training session lasting over 30 min. Moreover, anxiety and depression reduction after aerobic exercise may be achieved with exercise intensity between 30 and 70% of maximal heart rate ( Weinberg and Gould, 2015 ). To achieve positive mood changes, an important role is played even by anaerobic activity, such as yoga, or in all PEs in which there is rhythmic abdominal breathing, enjoyment, rhythmic, and repetitive movements and relative absence of interpersonal competition ( Berger and Motl, 2001 ).
PE determines positive biological and psychological effects that affect the brain and the cognitive functioning and promote a condition of wellbeing. PE plays an important role in counteract normal and pathological aging. Recent evidences have shown that PE triggers potent neuroplastic phenomena, partly mediated by epigenetic mechanisms. In fact, PE cause profound alterations in gene expression and its protein products in the form of epigenomic manifestations ( Fernandes et al., 2017 ).
A growing body of literature indicates that both chronic and aerobic PE can achieve similar benefits.
These results should lead to reflect on beneficial effects of PE and to promote its use as a modifiable factor for prevention, to improve cognitive abilities and to enhance mood.
Despite all these positive effects, it must be underlined that PE should be tailored to the individual. In fact, even PE, when excessive, can have a dark side, when PE becomes compulsive and facilitates addictive behaviors.
Author Contributions
LM, AP, SM, FF, GF, PS, and GS: designed the review; LM and GS: wrote the paper. All authors read, revised, and approved the final manuscript.
The present paper was supported by University of Naples Parthenope Ricerca Competitiva 2017 (D.R. 289/2017) to LM and GF.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adlard, P. A., Perreau, V. M., Pop, V., and Cotman, C. W. (2005). Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J. Neurosci. 25, 4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005
PubMed Abstract | CrossRef Full Text | Google Scholar
Akhavan, M. M., Emami-Abarghoie, M., Safari, M., Sadighi-Moghaddam, B., Vafaei, A. A., Bandegi, A. R., et al. (2008). Serotonergic and noradrenergic lesions suppress the enhancing effect of maternal exercise during pregnancy on learning and memory in rat pups. Neuroscience 151, 1173–1183. doi: 10.1016/j.neuroscience.2007.10.051
Aksu, I., Baykara, B., Ozbal, S., Cetin, F., Sisman, A. R., Dayi, A., et al. (2012). Maternal treadmill exercise during pregnancy decreases anxiety and increases prefrontal cortex VEGF and BDNF levels of rat pups in early and late periods of life. Neurosci. Lett. 516, 221–225. doi: 10.1016/j.neulet.2012.03.091
Andreazza, A. C., Wang, J. F., Salmasi, F., Shao, L., and Young, L. T. (2013). Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J. Neurochem. 127, 552–561. doi: 10.1111/jnc.12316
Asensio, S., Morales, J. L., Senabre, I., Romero, M. J., Beltran, M. A., Flores-Bellver, M., et al. (2016). Magnetic resonance imaging structural alterations in brain of alcohol abusers and its association with impulsivity. Addict. Biol. 21, 962–971. doi: 10.1111/adb.12257
Barbas, H. (2000). Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 52, 319–330. doi: 10.1016/S0361-9230(99)00245-2
Barrett, R. M., and Wood, M. A. (2008). Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 15, 460–467. doi: 10.1101/lm.917508
Bartholomew, J. B., Morrison, D., and Ciccolo, J. T. (2005). Effects of acute exercise on mood and well-being in patients with major depressive disorder. Med. Sci. Sport. Exerc. 37, 2032–2037. doi: 10.1249/01.mss.0000178101.78322.dd
Bartlett, J. D., Close, G. L., MacLaren, D. P. M., Gregson, W., Drust, B., and Morton, J. P. (2011). High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J. Sports Sci. 29, 547–553. doi: 10.1080/02640414.2010.545427
Basso, J. C., and Suzuki, W. A. (2017). The effects of acute exercise on mood, cognition, neurophysiology and neurochemical pathways: a review. Brain Plast. 2, 127–152. doi: 10.3233/BPL-160040
CrossRef Full Text | Google Scholar
Bavelier, D., and Neville, H. J. (2002). Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 3, 443–452. doi: 10.1038/nrn848
Berger, B., and Motl, R. (2001). “Physical activity and quality of life,” in Handbook of Sport Psychology , eds R. N. Singer, H. A. Hausenblas, and C. Janelle (New York, NY: Wiley), 636–670.
Google Scholar
Berger, B., and Tobar, D. (2011). “Exercise and quality of life,” in The NEW Sport and Exercise Psychology Companion , eds T. Morris and P. Terry (Morgantown, WV: Fitness Information Technology), 483–505.
Biddle, S. J. H., Atkin, A. J., Cavill, N., and Foster, C. (2011). Correlates of physical activity in youth: a review of quantitative systematic reviews. Int. Rev. Sport Exerc. Psychol. 4, 25–49. doi: 10.1080/1750984X.2010.548528
Black, J. E., Isaacs, K. R., Anderson, B. J., Alcantara, A. A., and Greenough, W. T. (1990). Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. U.S.A . 87, 5568–5572. doi: 10.1073/pnas.87.14.5568
Brown, B. M., Peiffer, J. J., Sohrabi, H. R., Mondal, A., Gupta, V. B., Rainey-Smith, S. R., et al. (2012). Intense physical activity is associated with cognitive performance in the elderly. Transl. Psychiatry 2:e191. doi: 10.1038/tp.2012.118
Brown, J., Cooper-Kuhn, C. M., Kempermann, G., Van Praag, H., Winkler, J., Gage, F. H., et al. (2003). Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x
Brunoni, A. R., Lopes, M., and Fregni, F. (2008). A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 11, 1169–1180. doi: 10.1017/S1461145708009309
Burkhalter, T. M., and Hillman, C. H. (2011). A narrative review of physical activity, nutrition, and obesity to cognition and scholastic performance across the human lifespan. Adv. Nutr. Int. Rev. J. 2, 201S−206S. doi: 10.3945/an.111.000331
Cabral, D. A., da Costa, K. G., Okano, A. H., Elsangedy, H. M., Rachetti, V. P., and Fontes, E. B. (2017). Improving cerebral oxygenation, cognition and autonomic nervous system control of a chronic alcohol abuser through a three-month running program. Addict. Behav. Rep. 6, 83–89. doi: 10.1016/j.abrep.2017.08.004
Carek, P. J., Laibstain, S. E., and Carek, S. M. (2011). Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 41, 15–28. doi: 10.2190/PM.41.1.c
Cataldo, A. M., McPhie, D. L., Lange, N. T., Punzell, S., Elmiligy, S., Ye, N. Z., et al. (2010). Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am. J. Pathol. 177, 575–585. doi: 10.2353/ajpath.2010.081068
Cechinel, L. R., Basso, C. G., Bertoldi, K., Schallenberger, B., de Meireles, L. C. F., and Siqueira, I. R. (2016). Treadmill exercise induces age and protocol-dependent epigenetic changes in prefrontal cortex of Wistar rats. Behav. Brain Res. 313, 82–87. doi: 10.1016/j.bbr.2016.07.016
Chaddock-Heyman, L., Erickson, K. I., Holtrop, J. L., Voss, M. W., Pontifex, M. B., Raine, L. B., et al. (2014). Aerobic fitness is associated with greater white matter integrity in children. Front. Hum. Neurosci. 8:584. doi: 10.3389/fnhum.2014.00584
Chang, M., Jonsson, P. V., Snaedal, J., Bjornsson, S., Saczynski, J. S., Aspelund, T., et al. (2010). The effect of midlife physical activity on cognitive function among older adults: ages—Reykjavik study. J. Gerontol. Ser. A 65A, 1369–1374. doi: 10.1093/gerona/glq152
Chang, Y. K., and Etnier, J. L. (2009). Exploring the dose-response relationship between resistance exercise intensity and cognitive function. J. Sport Exerc. Psychol. 31, 640–656. doi: 10.1123/jsep.31.5.640
Chang, Y. K., Labban, J. D., Gapin, J. I., and Etnier, J. L. (2012). The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 1453, 87–101. doi: 10.1016/j.brainres.2012.02.068
Chang, Y. K., Tsai, C. L., Hung, T. M., So, E. C., Chen, F. T., and Etnier, J. L. (2011). Effects of acute exercise on executive function: a study with a tower of London task. J. Sport Exerc. Psychol. 33, 847–865. doi: 10.1123/jsep.33.6.847
Chieffi, S., Messina, G., Villano, I., Messina, A., Valenzano, A., Moscatelli, F., et al. (2017). Neuroprotective effects of physical activity: evidence from human and animal studies. Front. Neurol. 8:188. doi: 10.3389/fneur.2017.00188
Coelho, F. G., de Gobbi, S., Andreatto, C. A. A., Corazza, D. I., Pedroso, R. V., and Santos-Galduróz, R. F. (2013). Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 56, 10–15. doi: 10.1016/j.archger.2012.06.003
Colberg, S. R., Somma, C. T., and Sechrist, S. R. (2008). Physical activity participation may offset some of the negative impact of diabetes on cognitive function. J. Am. Med. Dir. Assoc. 9, 434–438. doi: 10.1016/j.jamda.2008.03.014
Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., McAuley, E., et al. (2006). Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1166–1170. doi: 10.1093/gerona/61.11.1166
Colcombe, S., and Kramer, A. F. (2003). Fitness effects on the cognitive function of older adults. Psychol. Sci. 14, 125–130. doi: 10.1111/1467-9280.t01-1-01430
Craft, L. L. (2005). Exercise and clinical depression: examining two psychological mechanisms. Psychol. Sport Exerc. 6, 151–171. doi: 10.1016/j.psychsport.2003.11.003
Craft, L. L., and Perna, F. M. (2004). The benefits of exercise for the clinically depressed. Prim. Care Companion J. Clin. Psychiatry 6, 104–111. doi: 10.4088/PCC.v06n0301
Cutuli, D., Berretta, E., Caporali, P., Sampedro-Piquero, P., De Bartolo, P., Laricchiuta, D., et al. (2018). Effects of pre-reproductive maternal enrichment on maternal care, offspring's play behavior and oxytocinergic neurons. Neuropharmacology. doi: 10.1016/j.neuropharm.2018.02.015. [Epub ahead of print].
Cutuli, D., Berretta, E., Pasqualini, G., De Bartolo, P., Caporali, P., Laricchiuta, D., et al. (2017). Influence of pre-reproductive maternal enrichment on coping response to stress and expression of c-Fos and glucocorticoid receptors in adolescent offspring. Front. Behav. Neurosci. 11:73. doi: 10.3389/fnbeh.2017.00073
DeBoer, L. B., Powers, M. B., Utschig, A. C., Otto, M. W., and Smits, J. A. J. (2012). Exploring exercise as an avenue for the treatment of anxiety disorders. Expert Rev. Neurother. 12, 1011–1022. doi: 10.1586/ern.12.73
Deeny, S. P., Poeppel, D., Zimmerman, J. B., Roth, S. M., Brandauer, J., Witkowski, S., et al. (2008). Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol. Psychol. 78, 179–187. doi: 10.1016/j.biopsycho.2008.02.007
Deibel, S. H., Zelinski, E. L., Keeley, R. J., Kovalchuk, O., and McDonald, R. J. (2015). Epigenetic alterations in the suprachiasmatic nucleus and hippocampus contribute to age-related cognitive decline. Oncotarget 6, 23181–23203. doi: 10.18632/oncotarget.4036
De Moor, M. H., Beem, A. L., Stubbe, J. H., Boomsma, D. I., and De Geus, E. J. C. (2006). Regular exercise, anxiety, depression and personality: a population-based study. Prev. Med. 42, 273–279. doi: 10.1016/j.ypmed.2005.12.002
de Souza Moura, A. M., Lamego, M. K., Paes, F., Rocha, N. B. F., Simoes-Silva, V., Rocha, S. A., et al. (2015). Comparison among aerobic exercise and other types of interventions to treat depression: a systematic review. CNS Neurol. Disord. Drug Targets 14, 1171–1183. doi: 10.2174/1871527315666151111120714
Dietrich, A., and McDaniel, W. F. (2004). Endocannabinoids and exercise. Br. J. Sports Med. 38, 536–541. doi: 10.1136/bjsm.2004.011718
Ding, Q., Vaynman, S., Akhavan, M., Ying, Z., and Gomez-Pinilla, F. (2006). Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140, 823–833. doi: 10.1016/j.neuroscience.2006.02.084
Donnelly, J. E., Hillman, C. H., Castelli, D., Etnier, J. L., Lee, S., Tomporowski, P., et al. (2016). Physical activity, fitness, cognitive function, and academic achievement in children. Med. Sci. Sport. Exerc. 48, 1197–1222. doi: 10.1249/MSS.0000000000000901
Douw, L., Nieboer, D., van Dijk, B. W., Stam, C. J., and Twisk, J. W. (2014). A healthy brain in a healthy body: brain network correlates of physical and mental fitness. PLoS ONE 9:e88202. doi: 10.1371/journal.pone.0088202
Ehninger, D., and Kempermann, G. (2003). Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb. Cortex 13, 845–851. doi: 10.1093/cercor/13.8.845
Ekstrand, J., Hellsten, J., and Tingström, A. (2008). Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci. Lett. 442, 203–207. doi: 10.1016/j.neulet.2008.06.085
Erickson, K. I., Miller, D. L., Weinstein, A. M., Akl, S. L., and Banducci, S. (2012). Physical activity and brain plasticity in late adulthood: a conceptual and comprehensive review. Ageing Res. 3, 34–47. doi: 10.4081/ar.2012.e6
Erickson, K. I., Prakash, R. S., Voss, M. W., Chaddock, L., Hu, L., Morris, K. S., et al. (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19, 1030–1039. doi: 10.1002/hipo.20547
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Farioli-Vecchioli, S., Sacchetti, S., di Robilant, N. V., and Cutuli, D. (2018). The role of physical exercise and omega-3 fatty acids in depressive illness in the elderly. Curr. Neuropharmacol. 16, 308–326. doi: 10.2174/1570159X15666170912113852
Fernandes, J., Arida, R. M., and Gomez-Pinilla, F. (2017). Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 80, 443–456. doi: 10.1016/j.neubiorev.2017.06.012
Ferreira-Vieira, T. H., Bastos, C. P., Pereira, G. S., Moreira, F. A., and Massensini, A. R. (2014). A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus 24, 79–88. doi: 10.1002/hipo.22206
Foster, P. P. (2015). Role of physical and mental training in brain network configuration. Front. Aging Neurosci. 7:117. doi: 10.3389/fnagi.2015.00117
Fox, K. R. (2000). Self-esteem, self-perceptions and exercise. Int. J. Sport Psychol. 31, 228–240.
Fuss, J., Steinle, J., Bindila, L., Auer, M. K., Kirchherr, H., Lutz, B., et al. (2015). A runner's high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. U.S.A. 112, 13105–13108. doi: 10.1073/pnas.1514996112
Gelfo, F., Mandolesi, L., Serra, L., Sorrentino, G., and Caltagirone, C. (2018). The neuroprotective effects of experience on cognitive functions: evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience 370, 218–235. doi: 10.1016/j.neuroscience.2017.07.065
Giesen, E. S., Deimel, H., and Bloch, W. (2015). Clinical exercise interventions in alcohol use disorders: a systematic review. J. Subst. Abuse Treat. 52, 1–9. doi: 10.1016/j.jsat.2014.12.001
Gomes da Silva, S., Araujo, B. H. S., Cossa, A. C., Scorza, F. A., Cavalheiro, E. A., Naffah-Mazzacoratti Mda, G., et al. (2010). Physical exercise in adolescence changes CB1 cannabinoid receptor expression in the rat brain. Neurochem. Int. 57, 492–496. doi: 10.1016/j.neuint.2010.07.001
Grazioli, E., Dimauro, I., Mercatelli, N., Wang, G., Pitsiladis, Y., Di Luigi, L., et al. (2017). Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC Genomics 18:802. doi: 10.1186/s12864-017-4193-5
Grego, F., Vallier, J. M., Collardeau, M., Rousseu, C., Cremieux, J., and Brisswalter, J. (2005). Influence of exercise duration and hydration status on cognitive function during prolonged cycling exercise. Int. J. Sports Med. 26, 27–33. doi: 10.1055/s-2004-817915
Haasova, M., Warren, F. C., Ussher, M., Janse Van Rensburg, K., Faulkner, G., Cropley, M., et al. (2013). The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction 108, 26–37. doi: 10.1111/j.1360-0443.2012.04034.x
Hillman, C. H., Erickson, K. I., and Kramer, A. F. (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 9, 58–65. doi: 10.1038/nrn2298
Hirase, H., and Shinohara, Y. (2014). Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 280, 282–298. doi: 10.1016/j.neuroscience.2014.09.031
Hollamby, A., Davelaar, E. J., and Cadar, D. (2017). Increased physical fitness is associated with higher executive functioning in people with dementia. Front. Public Heal. 5:346. doi: 10.3389/fpubh.2017.00346
Hötting, K., and Röder, B. (2013). Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 37, 2243–2257. doi: 10.1016/j.neubiorev.2013.04.005
Hötting, K., Schickert, N., Kaiser, J., Röder, B., and Schmidt-Kassow, M. (2016). The effects of acute physical exercise on memory, peripheral bdnf, and cortisol in young adults. Neural Plast. 2016, 1–12. doi: 10.1155/2016/6860573
Hu, T., Zhou, F. J., Chang, Y. F., Li, Y. S., Liu, G. C., Hong, Y., et al. (2015). miR21 is associated with the cognitive improvement following voluntary running wheel exercise in TBI mice. J. Mol. Neurosci. 57, 114–122. doi: 10.1007/s12031-015-0584-8
Huang, P., Fang, R., Li, B. Y., and Chen, S.-D. (2016). Exercise-related changes of networks in aging and mild cognitive impairment brain. Front. Aging Neurosci. 8:47. doi: 10.3389/fnagi.2016.00047
Ieraci, A., Mallei, A., Musazzi, L., and Popoli, M. (2015). Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 25, 1380–1392. doi: 10.1002/hipo.22458
Isaacs, K. R., Anderson, B. J., Alcantara, A. A., Black, J. E., and Greenough, W. T. (1992). Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J. Cereb. Blood Flow Metab. 12, 110–119. doi: 10.1038/jcbfm.1992.14
Ji, E. S., Kim, Y. M., Shin, M. S., Kim, C. J., Lee, K. S., Kim, K., et al. (2015). Treadmill exercise enhances spatial learning ability through suppressing hippocampal apoptosis in Huntington's disease rats. J. Exerc. Rehabil. 11, 133–139. doi: 10.12965/jer.150212
Kim, S., and Kaang, B.-K. (2017). Epigenetic regulation and chromatin remodeling in learning and memory. Exp. Mol. Med. 49:e281. doi: 10.1038/emm.2016.140
Kleim, J. A., Cooper, N. R., and VandenBerg, P. M. (2002). Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 934, 1–6. doi: 10.1016/S0006-8993(02)02239-4
Knapen, J., Sommerijns, E., Vancampfort, D., Sienaert, P., Pieters, G., Haake, P., et al. (2009). State anxiety and subjective well-being responses to acute bouts of aerobic exercise in patients with depressive and anxiety disorders. Br. J. Sports Med. 43, 756–759. doi: 10.1136/bjsm.2008.052654
Korb, A., Bonetti, L. V., Da Silva, S. A., Marcuzzo, S., Ilha, J., Bertagnolli, M., et al. (2010). Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem. Res. 35, 380–389. doi: 10.1007/s11064-009-0066-x
Kou, X., Li, J., Liu, X., Chang, J., Zhao, Q., Jia, S., et al. (2017). Swimming attenuates d-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J. Appl. Physiol. 122, 1462–1469. doi: 10.1152/japplphysiol.00018.2017
Kramer, A. F., Hahn, S., Cohen, N. J., Banich, M. T., McAuley, E., Harrison, C. R., et al. (1999). Ageing, fitness and neurocognitive function. Nature 400, 418–419. doi: 10.1038/22682
Kronenberg, G., Bick-Sander, A., Bunk, E., Wolf, C., Ehninger, D., and Kempermann, G. (2006). Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging 27, 1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016
Lafenetre, P., Leske, O., Wahle, P., and Heumann, R. (2011). The beneficial effects of physical activity on impaired adult neurogenesis and cognitive performance. Front. Neurosci. 5:51. doi: 10.3389/fnins.2011.00051
Lambourne, K., and Tomporowski, P. (2010). The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 1341, 12–24. doi: 10.1016/j.brainres.2010.03.091
Landers, D. M., and Arent, S. M. (2001). “Physical activity and mental health,” in Handbook of Sport Psychology , eds R. N. Singer, H. A. Hausenblas, and C. Janelle (New York, NY: Wiley), 740–765.
Langdon, K. D., and Corbett, D. (2012). Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil. Neural Repair 26, 523–532. doi: 10.1177/1545968311425919
Lees, C., and Hopkins, J. (2013). Effect of aerobic exercise on cognition, academic achievement, and psychosocial function in children: a systematic review of randomized control trials. Prev. Chronic Dis. 10:130010. doi: 10.5888/pcd10.130010
Lin, T. W., and Kuo, Y. M. (2013). Exercise benefits brain function: the monoamine connection. Brain Sci. 3, 39–53. doi: 10.3390/brainsci3010039
Lin, T. W., Shih, Y. H., Chen, S. J., Lien, C. H., Chang, C. Y., Huang, T. Y., et al. (2015). Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 118, 189–197. doi: 10.1016/j.nlm.2014.12.005
Lista, I., and Sorrentino, G. (2010). Biological mechanisms of physical activity in preventing cognitive decline. Cell. Mol. Neurobiol. 30, 493–503. doi: 10.1007/s10571-009-9488-x
Loprinzi, P. D., Frith, E., Edwards, M. K., Sng, E., and Ashpole, N. (2018). The effects of exercise on memory function among young to middle-aged adults: systematic review and recommendations for future research. Am. J. Heal. Promot. 32, 691–704. doi: 10.1177/0890117117737409
Ludyga, S., Gerber, M., Brand, S., Holsboer-Trachsler, E., and Pühse, U. (2016). Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology 53, 1611–1626. doi: 10.1111/psyp.12736
Maejima, H., Kanemura, N., Kokubun, T., Murata, K., and Takayanagi, K. (2018). Exercise enhances cognitive function and neurotrophin expression in the hippocampus accompanied by changes in epigenetic programming in senescence-accelerated mice. Neurosci. Lett. 665, 67–73. doi: 10.1016/j.neulet.2017.11.023
Mammen, G., and Faulkner, G. (2013). Physical activity and the prevention of depression: a systematic review of prospective studies. Am. J. Prev. Med. 45, 649–657. doi: 10.1016/j.amepre.2013.08.001
Mandolesi, L., Gelfo, F., Serra, L., Montuori, S., Polverino, A., Curcio, G., et al. (2017). Environmental factors promoting neural plasticity: insights from animal and human studies. Neural Plast. 2017, 1–10. doi: 10.1155/2017/7219461
Marsh, H. W., and Sonstroem, R. J. (1995). Importance ratings and specific components of physical self-concept: relevance to predicting global components of self-concept and exercise. J. Sport Exerc. Psychol. 17, 84–104. doi: 10.1123/jsep.17.1.84
Martinsen, E. W. (1990). Benefits of exercise for the treatment of depression. Sports Med. 9, 380–389. doi: 10.2165/00007256-199009060-00006
Meyer, J. D., Ellingson, L. D., Koltyn, K. F., Stegner, A. J., Kim, J. S., and Cook, D. B. (2016a). Psychobiological responses to preferred and prescribed intensity exercise in major depressive disorder. Med. Sci. Sports Exerc. 48, 2207–2215. doi: 10.1249/MSS.0000000000001022
Meyer, J. D., Koltyn, K. F., Stegner, A. J., Kim, J.-S., and Cook, D. B. (2016b). Relationships between serum BDNF and the antidepressant effect of acute exercise in depressed women. Psychoneuroendocrinology 74, 286–294. doi: 10.1016/j.psyneuen.2016.09.022
Molteni, R., Ying, Z., and Gómez-Pinilla, F. (2002). Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur. J. Neurosci. 16, 1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x
Niemann, C., Godde, B., Staudinger, U. M., and Voelcker-Rehage, C. (2014). Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience 281, 147–163. doi: 10.1016/j.neuroscience.2014.09.033
Nithianantharajah, J., and Hannan, A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709. doi: 10.1038/nrn1970
Pahng, A. R., McGinn, M. A., and Paulsen, R. I. (2017). The prefrontal cortex as a critical gate of negative affect and motivation in alcohol use disorder. Curr. Opin. Behav. Sci. 13, 139–143. doi: 10.1016/j.cobeha.2016.11.004
Pan-Vazquez, A., Rye, N., Ameri, M., McSparron, B., Smallwood, G., Bickerdyke, J., et al. (2015). Impact of voluntary exercise and housing conditions on hippocampal glucocorticoid receptor, miR-124 and anxiety. Mol. Brain 8:40. doi: 10.1186/s13041-015-0128-8
Pedrinolla, A., Schena, F., and Venturelli, M. (2017). Resilience to Alzheimer's disease: the role of physical activity. Curr. Alzheimer Res. 14, 546–553.
Penedo, F. J., and Dahn, J. R. (2005). Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatry 18, 189–193. doi: 10.1097/00001504-200503000-00013
Pereira, A. C., Huddleston, D. E., Brickman, A. M., Sosunov, A. A., Hen, R., McKhann, G. M., et al. (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 104, 5638–5643. doi: 10.1073/pnas.0611721104
Petrosini, L., De Bartolo, P., Foti, F., Gelfo, F., Cutuli, D., Leggio, M. G., et al. (2009). On whether the environmental enrichment may provide cognitive and brain reserves. Brain Res. Rev. 61, 221–239. doi: 10.1016/j.brainresrev.2009.07.002
Querido, J. S., and Sheel, A. W. (2007). Regulation of cerebral blood flow during exercise. Sport. Med. 37, 765–782. doi: 10.2165/00007256-200737090-00002
Raedeke, T. D. (2007). The relationship between enjoyment and affective responses to exercise. J. Appl. Sport Psychol. 19, 105–115. doi: 10.1080/10413200601113638
Richter, H., Ambrée, O., Lewejohann, L., Herring, A., Keyvani, K., Paulus, W., et al. (2008). Wheel-running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav. Brain Res. 190, 74–84. doi: 10.1016/j.bbr.2008.02.005
Robinson, A. M., Eggleston, R. L., and Bucci, D. J. (2012). Physical exercise and catecholamine reuptake inhibitors affect orienting behavior and social interaction in a rat model of attention-deficit/hyperactivity disorder. Behav. Neurosci. 126, 762–771. doi: 10.1037/a0030488
Rodgers, W. M., Markland, D., Selzler, A. M., Murray, T. C., and Wilson, P. M. (2014). Distinguishing perceived competence and self-efficacy: an example from exercise. Res. Q. Exerc. Sport 85, 527–539. doi: 10.1080/02701367.2014.961050
Saab, B. J., and Mansuy, I. M. (2014). Neuroepigenetics of memory formation and impairment: the role of microRNAs. Neuropharmacology 80, 61–69. doi: 10.1016/j.neuropharm.2014.01.026
Sachs, M. L. (1981). “Running addiction,” in Psychology of Running , eds M. H. Sachs and M. L. Sachs (Champaign, IL: Human Kinetics), 116–127.
Schwellnus, M., Soligard, T., Alonso, J.-M., Bahr, R., Clarsen, B., Dijkstra, H. P., et al. (2016). How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 50, 1043–1052. doi: 10.1136/bjsports-2016-096572
Scully, D., Kremer, J., Meade, M. M., Graham, R., and Dudgeon, K. (1998). Physical exercise and psychological well being: a critical review. Br. J. Sports Med. 32, 111–120. doi: 10.1136/bjsm.32.2.111
Serra, L., Cercignani, M., Petrosini, L., Basile, B., Perri, R., Fadda, L., et al. (2011). Neuroanatomical correlates of cognitive reserve in alzheimer disease. Rejuvenation Res. 14, 143–151. doi: 10.1089/rej.2010.1103
Short, A. K., Yeshurun, S., Powell, R., Perreau, V. M., Fox, A., Kim, J. H., et al. (2017). Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl. Psychiatry 7:e1114. doi: 10.1038/tp.2017.82
Sibley, B. A., and Etnier, J. L. (2003). The relationship between physical activity and cognition in children: a meta-analysis. Pediatr. Exerc. Sci. 15, 243–256. doi: 10.1123/pes.15.3.243
Sim, Y. J. (2014). Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer's disease rats. J. Exerc. Rehabil. 10, 81–88. doi: 10.12965/jer.140102
Snigdha, S., de Rivera, C., Milgram, N. W., and Cotman, C. W. (2014). Exercise enhances memory consolidation in the aging brain. Front. Aging Neurosci. 6:3. doi: 10.3389/fnagi.2014.00003
Steiner, B., Kronenberg, G., Jessberger, S., Brandt, M. D., Reuter, K., and Kempermann, G. (2004). Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46, 41–52. doi: 10.1002/glia.10337
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/S1355617702813248
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6
Stessman, J., Hammerman-Rozenberg, R., Cohen, A., Ein-Mor, E., and Jacobs, J. M. (2009). Physical activity, function, and longevity among the very old. Arch. Intern. Med. 169, 1476–1483. doi: 10.1001/archinternmed.2009.248
Swain, R. A., Harris, A. B., Wiener, E. C., Dutka, M. V., Morris, H. D., Theien, B. E., et al. (2003). Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117, 1037–1046. doi: 10.1016/S0306-4522(02)00664-4
Thomas, S. N., Schroeder, T., Secher, N. H., and Mitchell, J. H. (1989). Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J. Appl. Physiol. 67, 744–748. doi: 10.1152/jappl.1989.67.2.744
Thomson, D., Turner, A., Lauder, S., Gigler, M. E., Berk, L., Singh, A. B., et al. (2015). A brief review of exercise, bipolar disorder, and mechanistic pathways. Front. Psychol. 6:147. doi: 10.3389/fpsyg.2015.00147
Tiryaki-Sonmez, G., Vatansever, S., Olcucu, B., and Schoenfeld, B. (2015). Obesity, food intake and exercise: relationship with ghrelin. Biomed. Hum. Kinet. 7, 116–124. doi: 10.1515/bhk-2015-0018
Tomporowski, P. D. (2003). Effects of acute bouts of exercise on cognition. Acta Psychol. 112, 297–324. doi: 10.1016/S0001-6918(02)00134-8
Urso, M. L., and Clarkson, P. M. (2003). Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189, 41–54. doi: 10.1016/S0300-483X(03)00151-3
van Praag, H. (2009). Exercise and the brain: something to chew on. Trends Neurosci. 32, 283–290. doi: 10.1016/j.tins.2008.12.007
van Praag, H., Christie, B. R., Sejnowski, T. J., and Gage, F. H. (1999a). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U.S.A. 96, 13427–13431.
PubMed Abstract | Google Scholar
van Praag, H., Kempermann, G., and Gage, F. H. (1999b). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270.
van Praag, H., Shubert, T., Zhao, C., and Gage, F. H. (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005
Vatansever-Ozen, S., Tiryaki-Sonmez, G., Bugdayci, G., and Ozen, G. (2011). The effects of exercise on food intake and hunger: relationship with acylated ghrelin and leptin. J. Sports Sci. Med. 10, 283–291.
Vaynman, S., Ying, Z., and Gomez-Pinilla, F. (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x
Voss, M. W., Chaddock, L., Kim, J. S., VanPatter, M., Pontifex, M. B., Raine, L. B., et al. (2011). Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience 199, 166–176. doi: 10.1016/j.neuroscience.2011.10.009
Waddington, C. H. (1939). An Introduction to Modern Genetics . London: George Allen and Unwin Ltd.
Wang, J., Fan, Y., Dong, Y., Ma, M., Ma, Y., Dong, Y., et al. (2016). Alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PLoS ONE 11:e0161956. doi: 10.1371/journal.pone.0161956
Weinberg, R. S., and Gould, D. (2015). Foundations of sport and exercise psychology, 6th Edn. Champaign, IL: Human Kinetics.
Windle, G., Hughes, D., Linck, P., Russell, I., and Woods, B. (2010). Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment. Health 14, 652–669. doi: 10.1080/13607861003713232
Winter, B., Breitenstein, C., Mooren, F. C., Voelker, K., Fobker, M., Lechtermann, A., et al. (2007). High impact running improves learning. Neurobiol. Learn. Mem. 87, 597–609. doi: 10.1016/j.nlm.2006.11.003
World Health Organization (2010). Global Recommendations on Physical Activity for Health . Geneva: WHO Press.
Yaffe, K., Fiocco, A. J., Lindquist, K., Vittinghoff, E., Simonsick, E. M., Newman, A. B., et al. (2009). Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology 72, 2029–2035. doi: 10.1212/WNL.0b013e3181a92c36
Young, S. N. (2007). How to increase serotonin in the human brain without drugs. J. Psychiatry Neurosci. 32, 394–399.
Zamani Sani, S. H., Fathirezaie, Z., Brand, S., Pühse, U., Holsboer-Trachsler, E., Gerber, M., et al. (2016). Physical activity and self-esteem: testing direct and indirect relationships associated with psychological and physical mechanisms. Neuropsychiatr. Dis. Treat. 12, 2617–2625. doi: 10.2147/NDT.S116811
Zanetti, M., Shigaeff, N., Menzes, A. H. T., and Takahashi, A. A. (2017). Cognitive reserve: evidence of delayed of dementia - a case report. J. Dement. 1:101.
Zubala, A., MacGillivray, S., Frost, H., Kroll, T., Skelton, D. A., Gavine, A., et al. (2017). Promotion of physical activity interventions for community dwelling older adults: a systematic review of reviews. PLoS ONE 12:e0180902. doi: 10.1371/journal.pone.0180902
Keywords: physical exercise, cognition, wellbeing, brain, epigenetic mechanisms
Citation: Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P and Sorrentino G (2018) Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol . 9:509. doi: 10.3389/fpsyg.2018.00509
Received: 04 January 2018; Accepted: 26 March 2018; Published: 27 April 2018.
Reviewed by:
Copyright © 2018 Mandolesi, Polverino, Montuori, Foti, Ferraioli, Sorrentino and Sorrentino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mandolesi, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The Future of Mental Health
Dispatches on the latest efforts in psychological and psychiatric treatment
How Exercise Boosts the Brain and Improves Mental Health
New research is revealing how physical activity can reduce and even ward off depression, anxiety and other psychological ailments
Bob Holmes, Knowable
:focal(800x602:801x603)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/1f/9a/1f9acb47-7f4e-499a-854c-1a93e355130f/gettyimages-861458264_web.jpg)
It’s hardly news that exercise is good for your physical health , and has long been extolled for mental health as well. But researchers are now making progress in understanding how, exactly, exercise may work its mental magic.
Exercise, they are learning, has profound effects on brain structure itself, and especially in regions most affected by depression and schizophrenia. It also provides other, more subtle benefits such as focus, a sense of accomplishment and sometimes social stimulation, all of which are therapeutic in their own right. And while more is generally better, even modest levels of physical activity, such as a daily walk, can pay big dividends for mental health.
“It’s a very potent intervention to be physically active,” says Anders Hovland, a clinical psychologist at the University of Bergen in Norway.
But that knowledge has barely begun to percolate into practice, says Joseph Firth, a mental health researcher at the University of Manchester in the UK. Just ask a hundred people receiving mental health care how many are getting exercise prescriptions as part of that care. “You wouldn’t find many,” Firth says.
Exercise — a tool against depression
Some of the strongest evidence for the mental benefits of exercise centers on depression. In 2016, Hovland and his colleagues searched the published literature and identified 23 clinical trials that tested the effectiveness of exercise in treating depression. Exercise was clearly effective and, in few studies, on par with antidepressant drugs, the researchers concluded.
And exercise offers several advantages. For one thing, antidepressant medications generally take several weeks or months to show their full effect. Exercise can improve mood almost immediately, making it a valuable supplement to frontline treatments such as drugs or therapy, notes Brett Gordon, an exercise psychology researcher at the Penn State College of Medicine. Plus, he says, exercise can counteract some of the unpleasant side effects of antidepressants, such as weight gain.
In addition, exercise has few of the negative side effects that are so common in drug therapies for depression and other disorders. “Many people who have mental health concerns are not enthusiastic about starting a medication for the rest of their lives, and are interested in pursuing other options. Exercise might be one of those options,” says Jacob Meyer, an exercise psychologist at Iowa State University.
There’s now emerging evidence that exercise also seems to help in treating or avoiding anxiety disorders , including post-traumatic stress disorder (PTSD), and possibly other serious psychotic conditions as well. “The more we do these studies, the more we see that exercise can be valuable,” says Firth.
There’s a flip side to this coin that’s especially relevant during the Covid-19 pandemic: If exercise stabilizes mental health, then anything that prevents people from working out is likely to destabilize it. To test this, Meyer and his colleagues surveyed more than 3,000 Americans about their activity before and during the pandemic. Those who became less active because of Covid reported more depression and poorer mental health , they found. (Ironically, those who had not exercised regularly pre-Covid didn’t report much change. “When you’re already at zero, where do you go?” says Meyer.)
But researchers are still figuring out exactly how muscular exertion acts on the brain to improve mental health. For most biomedical questions like this, the first stop is animal experiments, but they aren’t as useful in studies of mental health issues. “Psychological health is so uniquely human that it can be hard to make a good jump from animal models,” says Meyer.
Exercise and a healthy brain
Scientists have come up with a few ideas about how exercise enhances mental health , says Patrick J. Smith , a psychologist and biostatistician at Duke University Medical Center in North Carolina, who wrote about the subject in the 2021 Annual Review of Medicine with his Duke colleague Rhonda M. Merwin. It doesn’t seem to have much to do with cardiovascular fitness or muscular strength — the most obvious benefits of exercise — since how hard a person can work out is only weakly associated with their psychological health. Something else must be going on that’s more important than mere fitness, says Smith.
One likely possibility is that exercise buffs up the brain as well as the body. Physical exercise triggers the release of a protein known as brain-derived neurotrophic factor (BDNF). BDNF is one of the key molecules that encourage the growth of new brain cells — including, possibly, in the hippocampus, a brain region important in memory and learning. Since the hippocampus tends to be smaller or distorted in people with depression, anxiety and schizophrenia, boosting BDNF through exercise may be one way physical activity might help manage these conditions.
Sure enough, studies show that people with depression have lower levels of BDNF — and, notably, one effect of antidepressant drugs is to increase production of that molecule. Researchers have not yet shown directly that the exercise-associated increase in BDNF is what reduces depressive symptoms, but it remains one of the most promising possibilities, says Hovland.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/92/ed/92edabe0-a7a0-4a2a-9acb-e0ecb60dc078/g-exercise-mental-magic-alt_web.jpg)
Exercise may also help anxiety disorders. The brain changes prompted by BDNF appears to enhance learning, which is an important part of some anti-anxiety therapies. This suggests that exercise may be a useful way of improving the effectiveness of such therapies. One of the standard therapies for PTSD, for example, involves exposing patients to the fear-causing stimulus in a safe environment, so that the patients learn to recalibrate their reactions to trauma-linked cues — and the better they learn, the more durable this response might be.
Kevin Crombie, an exercise neuroscientist now at the University of Texas at Austin, and his colleagues tested this idea in the lab with 35 women with PTSD. Researchers first taught the volunteers to associate a particular geometric shape with a mild electric shock. The next day, the volunteers repeatedly saw the same shape without the shock, so that they would learn that the stimulus was now safe. A few minutes later, half the volunteers did 30 minutes of moderate exercise — jogging or uphill walking on a treadmill — while the other half did only light movement, not enough to breathe heavily.
The following day, those who had exercised were less likely to anticipate a shock when they saw the “trigger” shape, Crombie found — a sign that they had learned to no longer associate the trigger with danger . Moreover, those volunteers who showed the greatest exercise-induced increases in BDNF also did best at this relearning.
Although the evidence is not yet definitive, a few studies have suggested that regular exercise may lead to better outcomes in patients with schizophrenia too. Vijay Mittal, a psychologist at Northwestern University, wondered whether working out also might prevent people from developing the disorder in the first place.
Mittal works with teenagers who are at high risk of psychotic disorders such as schizophrenia, but who have not yet progressed to the full-on disorder. In the past two decades, researchers have gotten much better at recognizing such individuals just as they begin to display the earliest signs of illness, such as seeing shadows out of the corner of their eye or hearing indistinct voices when no one is home.
For about 10 percent to 33 percent of these teens, these early signs turn into something more serious. “A shadow might turn into a person,” says Mittal. “A whisper might turn into words. A suspicion that someone is following them might turn into the belief that the government is after them.”
Previously, Mittal had found that the hippocampus is different in at-risk teens who later slid down this slippery slope than in those who didn’t. He wondered whether exercise might help bolster the hippocampus and avert the slide. So his team tested this notion in a sample of 30 high-risk teens, half of whom followed a regimen of aerobic exercise twice a week for three months. (The other half, the control group, were told they were on the wait list for the exercise program.) The researchers used brain scans to look at participants’ hippocampus before and after the program.
The experiment has just concluded, and Mittal is still analyzing the results, which he calls promising. He cautions, however, that exercise is not a panacea. Schizophrenia is a diverse disease, and even if physical activity proves to be protective for some at-risk people, not all are likely to respond to it in the same way. “It’s really important to remember that these disorders are complicated. I’ve worked with people who exercise a lot and still have schizophrenia,” he says.
If Mittal’s work pans out, exercise may allow mental health workers to help a group that isn’t easily treated with drugs. “Someone who’s just at risk of a psychotic disorder, you can’t just give them medication, because that has risks too,” says Firth. “It would be fantastic to have something else in our arsenal.”
Exercise almost certainly has other effects on the brain, too, experts agree. For example, exercise stimulates the release of endocannabinoids, molecules that are important in modifying the connections between brain cells, the mechanism that underlies learning. This may provide another way of enhancing the learning that underlies successful treatment for depression, PTSD and other mental disorders. Indeed, Crombie’s study of exercise in a simplified model of PTSD therapy measured endocannabinoids as well as BDNF, and increases in both were associated with stronger learning responses.
And physical activity also moderates the body’s response to stress and reduces inflammation, both of which could plausibly help improve brain health in people with mental illness. “We have just scratched the surface,” says Hovland.
Moving the body, engaging the mind
But changing the structure of the brain isn’t the only way physical activity can be beneficial for those suffering from mental health conditions. The habit of exercise can itself be beneficial, by altering people’s thought patterns, says Smith.
For people with mental health issues, simply doing something — anything — can be helpful in its own right because it occupies their attention and keeps them from ruminating on their condition. Indeed, one survey of the published literature found that placebo exercise — that is, gentle stretching, too mild to cause any physiological effect — had almost half the beneficial effect on mental health as strenuous exercise did.
Besides just occupying the mind, regular workouts also give exercisers a clear sense of progress as their strength and fitness improves. This sense of accomplishment — which may be especially notable for weight training, where people make quick, easily measurable gains — can help offset some of the burden of anxiety and depression, says Gordon.
If so, playing a musical instrument, studying a language and many other activities could also help people cope with mental health conditions in a similar way. But exercise does more than that, making it one of the best choices for managing mental health. “You can see benefits from doing anything, but the exercise may confer greater benefits,” says Firth.
For one thing, relatively strenuous exercise teaches people to put up with short-term discomfort for long-term gain. People who suffer from anxiety disorders such as PTSD or panic attacks often show a reduced ability to tolerate mental discomfort, so that experiences most people would cope with result in uncontrolled anxiety instead. There’s now emerging evidence that regular exercise builds tolerance for internal discomfort, and this may explain part of its benefit in managing these conditions, Smith says.
Using exercise as a mental health treatment brings some challenges, however. “People with mental illness are also at higher risk of struggling with low motivation,” Firth says. This can make it difficult to organize and stick with an exercise program, and many patients need additional support.
This is often difficult, because psychologists, psychiatrists and other mental health workers are often already overburdened. Plus, prescribing and supervising exercise hasn’t traditionally been within these practitioners’ purview. “We’re telling people, ‘Hey, exercise is helpful,’ but we’re telling it to people who can’t really incorporate it because they often don’t get any training,” Firth says. Exercise referral schemes, which link patients with fitness specialists and structured programs at community leisure centers, have been used in the UK and other places to encourage exercise in people with physical conditions such as obesity and diabetes. A similar approach could be valuable for mental health conditions, Firth says.
Therapists can also help patients persist for the long term by tailoring their exercise prescriptions to each individual’s capabilities. “I always tell patients that doing anything is better than doing nothing, and the best exercise for you is the one you’ll actually do,” Smith says.
The secret, he suggests, is to make sure people stop exercising before they’ve done so much it makes them feel exhausted afterward. “When you feel like crap after exercise, you’re not going to want to do it,” he says, because the brain tags the activity as something unpleasant. It’s far better to have the patient quit while they still have a positive feeling from the workout. “Without even realizing it, their brain tends to tag that activity as something more pleasurable. They don’t dread it.”
Even light activity — basically just moving around now and then during the day instead of sitting for hours at a time — may help. In one study of more than 4,000 adolescents in the UK, Aaron Kandola, a psychiatric epidemiologist at University College London, and his colleagues found that youths who undertook more light activity during the day had a lower risk of depressive symptoms than those who spent more time sitting.
“What we really need are big exercise trials where we compare different amounts against each other,” says Kandola. “Instead, what we have are different studies that used different amounts of activity.” That makes precise recommendations difficult, because each study varies in terms of its patient populations and methods, and follows results for a different length of time. As researchers learn more about the mechanisms linking exercise to mental health, they should be able to refine their exercise prescriptions so that patients are best able to manage their illnesses.
And exercise has powerful benefits for people with mental illness that go beyond its effects on the illnesses themselves. Many struggle with related issues such as social withdrawal and a reduced capacity for pleasure, says Firth. Standard medications reduce some symptoms but do nothing to address these other problems. Exercise — especially as part of a group — can help boost their mood and enrich their lives.
Even more important, people with serious mental illnesses such as severe depression and schizophrenia also are more likely to have significant physical health issues such as obesity, heart disease and other chronic diseases, and as a result their life expectancy is 10 to 25 years lower than that of unaffected people.
“Reducing those health risks is really paramount at the moment,” says Kandola. “That’s the big appeal of exercise: We already know it can improve physical health. If it does have mental health benefits as well, it can be quite an important addition to treatment.”
Knowable Magazine is an independent journalistic endeavor from Annual Reviews.
Get the latest Science stories in your inbox.

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Exercise and physical activity
Physical activity is an important part of healthy aging. Check out these articles for the latest on how exercise and physical activity can help you stay healthy as you age. Find tips on how to fit exercise into your daily life safely and get motivated to get moving!
5 Tips to Help You Stay Motivated to Exercise

Exercise and Physical Activity Worksheets

Exercising With Chronic Conditions

Finding the Right Fitness Shoes and Clothes

Four Types of Exercise Can Improve Your Health and Physical Ability

Four Types of Exercise and Physical Activity

Fun Ways for Older Adults to Stay Physically Active

Get Fit for Free

How Older Adults Can Get Started With Exercise

No More Excuses! Overcome Exercise Barriers

nia.nih.gov
An official website of the National Institutes of Health
Benefits of exercise
Step right up! It's the miracle cure we've all been waiting for.
It can reduce your risk of major illnesses, such as coronary heart disease , stroke , type 2 diabetes and cancer and lower your risk of early death by up to 30%.
It's free, easy to take, has an immediate effect and you don't need a GP to get some. Its name? Exercise.
Check physical activity guidelines for:
- children (under 5 years)
- children and young people (5 to 18 years)
- exercise (adults 19 to 64 years)
- older adults (65 years and over)
Exercise is the miracle cure we've always had, but for too long we've neglected to take our recommended dose. Our health is now suffering as a consequence.
This is no snake oil. Whatever your age, there's strong scientific evidence that being physically active can help you lead a healthier and happier life.
People who exercise regularly have a lower risk of developing many long-term (chronic) conditions, such as heart disease, type 2 diabetes, stroke, and some cancers.
Research shows that physical activity can also boost self-esteem, mood, sleep quality and energy, as well as reducing your risk of stress , clinical depression , dementia and Alzheimer's disease .
Health benefits
Given the overwhelming evidence, it seems obvious that we should all be physically active. It's essential if you want to live a healthy and fulfilling life into old age.
It's medically proven that people who do regular physical activity have lower risk of:
- coronary heart disease and stroke
- type 2 diabetes
- bowel cancer
- breast cancer in women
- early death
- osteoarthritis
- hip fracture
- falls (among older adults)
What counts?
To stay healthy, the UK Chief Medical Officers' Physical Activity Guidelines, on GOV.UK , state that adults should try to be active every day and aim to do at least 150 minutes of physical activity over a week, through a variety of activities.
For most people, the easiest way to get moving is to make activity part of everyday life, like walking for health or cycling instead of using the car to get around. However, the more you do, the better, and taking part in activities such as sports and exercise will make you even healthier.
For any type of activity to benefit your health, you need to be moving quick enough to raise your heart rate, breathe faster and feel warmer. This level of effort is called moderate intensity activity. If you're working at a moderate intensity you should still be able to talk but you won't be able to sing the words to a song.
An activity where you have to work even harder is called vigorous intensity activity. There is substantial evidence that vigorous activity can bring health benefits over and above that of moderate activity. You can tell when it's vigorous activity because you're breathing hard and fast, and your heart rate has gone up quite a bit. If you're working at this level, you won't be able to say more than a few words without pausing for a breath.
Video: Keep healthy with 150 minutes of exercise a week
In this video people describe what exercise they do, including cycling, running and swimming.
A modern problem
People are less active nowadays, partly because technology has made our lives easier. We drive cars or take public transport. Machines wash our clothes. We entertain ourselves in front of a TV or computer screen. Fewer people are doing manual work, and most of us have jobs that involve little physical effort. Work, household chores, shopping and other necessary activities are far less demanding than for previous generations.
We move around less and burn off less energy than people used to. Research suggests that many adults spend more than 7 hours a day sitting down, at work, on transport or in their leisure time. People aged over 65 spend 10 hours or more each day sitting or lying down, making them the most sedentary age group.
Sedentary lifestyles
Inactivity is described by the Department of Health and Social Care as a "silent killer". Evidence is emerging that sedentary behaviour, such as sitting or lying down for long periods, is bad for your health.
Not only should you try to raise your activity levels, but you should also reduce the amount of time you and your family spend sitting down.
Common examples of sedentary behaviour include watching TV, using a computer, using the car for short journeys and sitting down to read, talk or listen to music. This type of behaviour is thought to increase your risk of developing many chronic diseases, such as heart disease, stroke and type 2 diabetes, as well as weight gain and obesity .
Crucially, you can hit your weekly activity target but still be at risk of ill health if you spend the rest of the time sitting or lying down.
For a summary on the health benefits of being more active, check out these physical activity guidelines from the Department of Health and Social Care .
Page last reviewed: 4 August 2021 Next review due: 4 August 2024
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Exercise and fitness effect on obesity.
Grace M. Niemiro ; Ayesan Rewane ; Amit M. Algotar .
Affiliations
Last Update: November 17, 2023 .
- Introduction
Obesity represents a significant public health concern, with one-third of adults classified as living with obesity in the United States. Obesity correlates with cardiometabolic comorbidities that can decrease the quality of life. [1] [2] Researchers have proposed that exercise is an important lifestyle measure to maintain a healthy weight. This review will cover the role of exercise in obesity and fitness.
Obesity is an excessive fat accumulation in adipose tissues defined by a body mass index (BMI) of 30 kg/m 2 and above. Individuals in the BMI range of 25 to 30 kg/m 2 are categorized as overweight, while a BMI of 40 kg/m 2 and above is regarded as morbid obesity. [3] Obesity correlates with an individual’s increased risk of cancers, stroke, metabolic disease, heart failure, and other cardiovascular conditions, highlighting the need to reduce the incidence and prevalence of obesity. [4] [5] [6] Chronic low-grade inflammation associated with obesity is hypothesized to have associations with adverse cardiometabolic adverse effects. [7] Although short-term inflammation is beneficial to initiate an immune response, chronically elevated levels of inflammation exhaust the immune system and contribute to immune dysfunction. [2] Researchers posit that this inflammation is stimulated by the excess adipose tissue, which has consistently been shown to play a role as an active endocrine organ. [8]
Reducing adipose tissue is one of the ways to reduce weight in individuals with obesity and is necessary to mitigate negative cardio-metabolic comorbidities in obesity. Two methods exist that can effectively decrease adipose tissue and include:
- Dietary modification
- Energy expenditure modification (ie, exercise)
Thus, increasing energy expenditure can help reduce excess adipose tissue and obesity. The current guidelines by the American College of Sports Medicine (ACSM) include aerobic or anaerobic exercise. Aerobic exercise (eg, running, cycling, rowing) is an exercise that exhausts the oxygen in the muscles. Still, oxygen consumption is sufficient to supply the energy demands placed on the muscles and does not need to derive energy from another source. [9] On the other hand, anaerobic exercise or resistance exercise, eg, weight lifting)is oxygen consumption insufficient to supply the energy demands placed on the muscles, and muscles must break down other energy supplies, such as sugars, to produce energy and lactic acid. [9] Physical activity is included in the exercise, although it does not necessarily include structured exercise plans/sessions.
The measurement of exercise is conducted in “metabolic equivalent tasks” (METs), which roughly equate to the effort and energy expenditure it takes for an individual to sit quietly. Physical activity is frequently incorporated into different lifestyle interventions, highlighting the need for regular daily physical activity. Physical activity in the general lifestyle includes goal setting, problem-solving, leisure-time physical activity, and activity used for commuting. Outcomes of interest include cardiorespiratory fitness, body composition, and muscular fitness. Recently, much literature has shown the positive effects of exercise on physical health and cognitive and emotional well-being in people of all ages. [10]
- Issues of Concern
Overweight and obese people can partake in the same exercise prescriptions as individuals with normal weight. Special considerations are essential, accounting for prevalent obesity-related comorbidities like orthopedic risks (eg, arthritis) and pulmonary and cardiac conditions. However, this should not deter individuals from participating in exercise programs, as exercise is essential for overall health. [11] [12] Currently, there are several exercise guidelines for individuals living with obesity, including the American College of Sports Medicine (ACSM), the Obesity Medical Association (OMA), and the Obesity Society (TOS), which are all clinically available to aid individuals in prescribing exercise. Here, we outline the general recommendations for individuals living with obesity as follows:
A . Patients must be cleared by their healthcare provider for any comorbid conditions by history and physical examination to maximize patient safety. [13] Examples include the Physical Activity Readiness Questionnaire (PAR-Q) and the Health/Fitness Facility Preparticipation Screening Questionnaire. [14] [15]
B . A minimum of 150 to 300 minutes of moderate physical activity per week or 75 to 150 minutes of vigorous physical activity weekly is essential to prevent weight regain, increase weight loss, and improve fitness. [14] However, for individuals who wish to lose weight, at least 200 to 300 minutes of moderate to vigorous physical activity each week is recommended to encourage long-term weight loss. [14] [15]
- The recommendation for inactive individuals is to “start low and go slow” by starting with lower-intensity activities and gradually increasing the frequency and duration of the activity.
- It is an excellent idea to spread out aerobic activity over the week versus all the time in one day.
- Utilize appropriate gear and sports equipment and choose safe environments.
- Adjust exercises to decrease orthopedic risk or is nonambulatory (if applicable). This can include cycling instead of running if an individual has arthritis. The exercise guidelines still apply if individuals are not ambulatory or may have to modify exercise due to particular circumstances. However, the patient can get creative to find ways to achieve them, such as utilizing more ambulatory limbs (eg, moving arms faster to get the heart rate up if legs cannot be used, upper body ergometer, etc.)
- Anaerobic training can be implemented and may even increase muscle mass. Anaerobic exercise is not practical in altering energy expenditure or absolute weight loss. [13] However, anaerobic exercise is highly encouraged if the patient's goal is to increase muscle mass. Furthermore, each muscle group should be exercised at least 10 sets per week to increase muscle mass, with one set of 8 to 10 reps. Also, ensure proper form to avoid injuries. Individuals who are not ambulatory or may have limited movement can still participate in an anaerobic exercise. Individuals must ensure proper form but can modify exercises as needed, such as upper body-only exercises, lower body-only exercises, using a neutral grip, keeping stable movements, etc.
- Clinical Significance
Utilizing exercise to reduce obesity (ie, reducing fat mass) has benefits beyond reducing fat mass. In many instances, fitness is associated with more desirable clinical outcomes, such as decreasing metabolic disease, cardiovascular disease, Alzheimer's disease risk, inflammation, and many other disease states not listed here. [14] [15] [16]
Exercise/physical activity is a proven modality for treating the disease of overweight and obesity. However, managing this disease is best through dietary interventions and regular exercise. Exercise is an integral part of not only weight loss but overall health as well. A balanced hypocaloric diet, aerobic training, and cognitive behavioral therapy (CBT) help reduce weight. Weight-reducing pharmacotherapy is indicated in individuals with a BMI greater than 30 kg/m2 with or without comorbidities. Bariatric surgery is only needed to reduce weight in BMI greater than 40 kg/m2, especially with comorbidity.
The Food Drug Administration (FDA) approved medications and their mechanism of action:
- Orlistat inhibits pancreatic gastric lipase
- The phentermine/topiramate combination is unknown, but it is believed to inhibit Norepinephrine (NE) release and GABA gamma-aminobutyric acid transmission
- Bupropion/naltrexone combination, NE/dopamine reuptake inhibitor (NDRI), naltrexone (an opioid antagonist)
- Liraglutide, a glucagon-like peptide- GLP-1 agonist, decreases dipeptidyl peptidase-IV metabolism and appetite.
Aerobic exercise is a form of physical activity proven to be efficacious in managing obesity. Moderate- or high-intensity aerobics involving larger groups of muscles is recommended. Aerobic exercise should be practiced for a long duration to appreciate the effect. Hence, a weekly aerobic exercise of at least 150 to 180 minutes can increase physical fitness. Resistance exercise has also been shown to have some meaningful impact on weight. [17] [18] [19] [20] [21]
- Enhancing Healthcare Team Outcomes
The healthcare team (nurse practitioner, primary care provider, internist, endocrinologist, bariatric surgeon, pharmacist, and obesity nurse) should implement many strategies to increase physical activity and fitness for individuals living with obesity, including utilizing “exercise vital signs,” tracking exercise, motivational interviewing, and periodic check-ins. Currently, the following could potentially be implemented into practice to encourage patients living with obesity to exercise.
Utilizing exercise as a vital sign in individuals with obesity: Obtaining current exercise and physical activity habits from patients could serve as another vital sign and would include understanding the intensity, mode, and duration of the exercise performed weekly by the patient. Providers could have electronic medical records (EMRs) to prompt patients who are living with obesity to have discussions with the patient regarding their physical activity. These prompts on the EMR can be input by the medical assistants who may ask at the beginning of the appointment, just like taking blood pressure and pulse.
Utilizing exercise trackers: Several devices can track heart rate, motion, exercise, moderate to vigorous physical activity (MVPA), and beyond. Providers could potentially use these data to ensure that the patient is exercising and could point to potential problems that may arise from abnormal heart or exercise responses. Examples include smartwatches, cellular smartphones, pedometers, heart rate monitors, etc.
Motivational Interviewing: To drive the point home further, nurses, CNAs, physicians, and anyone else involved in the healthcare setting for this patient could employ/use motivational interviewing techniques with the patient to reflect, plan, and execute different action plans to ensure that patients are meeting their exercise goals.
Check-Ins: Technology is allowing individuals to interact now more than ever. Physicians and patients could potentially use these technological advances to develop relationships further. Utilizing technology to have doctor-patient check-ins regarding their exercise may increase the adherence of obese individuals to exercise programs. These could include developing an app that alerts patients and the doctor when exercise habits are not sufficient, thus prompting a check-in from the physician using motivational interviewing and asking why the patient has or hasn’t exercised according to plan.
- Nursing, Allied Health, and Interprofessional Team Interventions
If the patient can exercise, exercise may be the preferred route to decrease disease symptoms and future risk compared to alternative pharmaceuticals that may exacerbate symptoms. An open and communicative relationship between the physician, healthcare team, and the patient must be present to suggest adding exercise to the patient's lifestyle to decrease obesity and improve adverse side effects. [22] Obesity disproportionately affects individuals with a lower socioeconomic status, and these individuals may not have access to a safe exercise space, may not understand the importance of exercise, or may not have the time during the day to exercise due to other obligations. Therefore, the relationship between the care providers and the patient becomes significant in implementing exercise in obese individuals.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Grace Niemiro declares no relevant financial relationships with ineligible companies.
Disclosure: Ayesan Rewane declares no relevant financial relationships with ineligible companies.
Disclosure: Amit Algotar declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Niemiro GM, Rewane A, Algotar AM. Exercise and Fitness Effect on Obesity. [Updated 2023 Nov 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: A systematic review. [JBI Libr Syst Rev. 2012] The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: A systematic review. Antwi F, Fazylova N, Garcon MC, Lopez L, Rubiano R, Slyer JT. JBI Libr Syst Rev. 2012; 10(42 Suppl):1-14.
- The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. [JBI Database System Rev Implem...] The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. Spencer L, Rollo M, Hauck Y, MacDonald-Wicks L, Wood L, Hutchesson M, Giglia R, Smith R, Collins C. JBI Database System Rev Implement Rep. 2015 Jan; 13(1):88-98.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Exercise in weight management of obesity. [Cardiol Clin. 2001] Review Exercise in weight management of obesity. Poirier P, Després JP. Cardiol Clin. 2001 Aug; 19(3):459-70.
- Review Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. [Cochrane Database Syst Rev. 2016] Review Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Cochrane Database Syst Rev. 2016 Mar 31; 3(3):CD008796. Epub 2016 Mar 31.
Recent Activity
- Exercise and Fitness Effect on Obesity - StatPearls Exercise and Fitness Effect on Obesity - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Physical exercise influences physiological, psychological, and genetical changes, which results in producing various benefits, including preventing metabolic and mood disorders. Aerobic exercise benefits on physiology among sedentary adults have also been evaluated with genetic markers. One such study involved participants in a 30-min ...
Background: The aim of the present work is the elaboration of a systematic review of existing research on physical fitness, self-efficacy for physical exercise, and quality of life in adulthood.Method: Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines, and based on the findings in 493 articles, the final sample was composed of 37 ...
Background Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity ...
2. Definitions of Physical Activity, Exercise, Training, Sport, and Health. Definitions and terms are based on "Physical activity in the prevention and treatment of disease" (FYSS, www.fyss.se [Swedish] []), World Health Organization (WHO) [] and the US Department of Human Services [].The definition of physical activity in FYSS is: "Physical activity is defined purely physiologically, as ...
Research has found that briskly walking for 450 minutes each week is associated with living around 4.5 years longer than doing no leisure-time exercise 1, and that engaging in regular physical ...
Since ancient times, the health benefits of regular physical activity/exercise have been recognized and the classic studies of Morris and Paffenbarger provided the epidemiological evidence in support of such an association. Cardiorespiratory fitness, often measured by maximal oxygen uptake, and habitual physical activity levels are inversely related to mortality. Thus, studies exploring the ...
A majority of reports show that physical exercise is associated with enhanced cognition throughout different populations and remains as a fascinating area in scientific research because of its ...
Abstract. Purpose of review: The health benefits of physical activity and exercise are clear; virtually everyone can benefit from becoming more physically active. Most international guidelines recommend a goal of 150 min/week of moderate-to-vigorous intensity physical activity. Many agencies have translated these recommendations to indicate ...
Substantial evidence has established the value of high levels of physical activity, exercise training (ET), and overall cardiorespiratory fitness in the prevention and treatment of cardiovascular diseases. This article reviews some basics of exercise physiology and the acute and chronic responses of ET, as well as the effect of physical ...
It is well known that exercise generally enhances cognitive function 1,2,3,4,5,6,7,8,9,10.However, the environment in which exercise is performed may be just as important as the exercise itself 11 ...
The good news is that even small amounts of physical activity can immediately reduce symptoms of anxiety in adults and older adults. Depression has also shown to be responsive to physical activity. Research suggests that increased physical activity, of any kind, can improve depression symptoms experienced by people across the lifespan.
Health is a state of complete physical, mental and social well-being and not merely absence of disease [ 1 ]. Fitness is an ability to execute daily functional activities with optimal performance, endurance, and strength to manage minimalist of disease, fatigue, stress and reduced sedentary behavior [ 2 ]. In the modern era with advancement in ...
To the author's knowledge, this concept has been adopted only in scientific (as opposed to epidemiological) studies. Its importance in the epidemiology of physical activity is evidenced by data from British civil servants. 12 Whereas only frequent vigorous exercise (defined as liable to entail peaks of energy expenditure of ≥7.5 kcal.min -1 [31.5 kJ.min -1]) was associated with ...
In The Lancet Psychiatry, Chekroud and colleagues1 presented a large cross-sectional examination of physical activity and mental health. Despite imprecision about the terms mental health and exercise in the study—and the cross-sectional design—the findings overall match the existing body of longitudinal research showing that regular physical activity is associated with better mental health.2
changes take place or have already taken place, such as menopause and andropause, which involve. diverse psychological impacts and, frequently, physiological changes. A loss of bone mass, for ...
We conducted a systematic review by searching latest 8 years publications. PubMed and Scopus were used to identify eligible studies with the searching terms, 'sleep quality' AND 'physical activity', within the timeframe between January 2010 and June 2018. All the included articles were systematically reviewed and analysed.
Physical Exercise, Brain, and Cognition. Among the biological effects of PE, those linked to "neuroplasticity" are quite important. Neuroplasticity is an important feature of the nervous system, which can modify itself in response to experience (Bavelier and Neville, 2002).For this reason, PE may be considered as an enhancer environmental factor promoting neuroplasticity.
Physical activity can help: Reduce feelings of depression and stress, while improving your mood and overall emotional well-being. Increase your energy level. Improve sleep. Empower you to feel more in control. In addition, exercise and physical activity may possibly improve or maintain some aspects of cognitive function, such as your ability to ...
New research is revealing how physical activity can reduce and even ward off depression, anxiety and other psychological ailments. Runners in Hawaii exercise at sunset. Exercise has profound ...
While future research should determine whether the FNDC5 cleavage-product was produced locally in hippocampal neurons or was secreted into the circulation, this finding eloquently displays one mechanism responsible for brain health benefits following exercise. ... Physical exercise prevents stress-induced activation of granule neurons and ...
Research suggests exercising in a park or other natural setting is more beneficial than exercising indoors. Health practitioners and fitness buffs have long known that regular physical activity ...
Aerobic exercise is a physical activity that uses your body's large muscle groups, is rhythmic and repetitive. It increases your heart rate and how much oxygen your body uses. Examples of aerobic exercises include walking, cycling and swimming. It reduces your risk of heart disease, diabetes, high blood pressure and high cholesterol.
The Special Issue "Physical Activity, Wellness and Health: Challenges, Benefits and Strategies" was intended to collect research articles on anthropometric determinants of health and performance, PA and healthy habits, exercise and diet, exercise and body composition, interventions to promote PA for people of all ages, strategies for the ...
Exercise and physical activity. Physical activity is an important part of healthy aging. Check out these articles for the latest on how exercise and physical activity can help you stay healthy as you age. Find tips on how to fit exercise into your daily life safely and get motivated to get moving!
People who exercise regularly have a lower risk of developing many long-term (chronic) conditions, such as heart disease, type 2 diabetes, stroke, and some cancers. Research shows that physical activity can also boost self-esteem, mood, sleep quality and energy, as well as reducing your risk of stress, clinical depression, dementia and ...
Future research should focus on easily accessible methods that reflect relevant markers of aerobic exercise intensity more appropriately, such as based on the ventilatory thresholds, and on the feasibility of an overarching measure for training load that relates to the FITT-characteristics. ... In conclusion, physical fitness training can be an ...
Exercise/physical activity is a proven modality for treating the disease of overweight and obesity. However, managing this disease is best through dietary interventions and regular exercise. Exercise is an integral part of not only weight loss but overall health as well. A balanced hypocaloric diet, aerobic training, and cognitive behavioral ...