| Vanadium forms a number of different ions - for example, V and V . If you think about how these might be produced from vanadium metal, the 2+ ion will be formed by oxidising the metal by removing two electrons: + 2e The vanadium is now said to be in an oxidation state of +2. Removal of another electron gives the V ion: + e The vanadium now has an oxidation state of +3. Removal of another electron gives a more unusual looking ion, VO . + H O + 2H + e The vanadium is now in an oxidation state of +4. Notice that the oxidation state isn't simply counting the charge on the ion (that was true for the first two cases but not for this one). The positive oxidation state is counting the total number of electrons which have had to be removed - starting from the element. It is also possible to remove a fifth electron to give another ion (easily confused with the one before!). The oxidation state of the vanadium is now +5. + H O + 2H + e Every time you oxidise the vanadium by removing another electron from it, its oxidation state increases by 1. Fairly obviously, if you start adding electrons again the oxidation state will fall. You could eventually get back to the element vanadium which would have an oxidation state of zero. What if you kept on adding electrons to the element? You can't actually do that with vanadium, but you can with an element like sulphur. The sulphur has an oxidation state of -2. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to an element (a negative oxidation state) to get to its present state. Recognising this simple pattern is the single most important thing about the concept of oxidation states. If you know how the oxidation state of an element changes during a reaction, you can instantly tell whether it is being oxidised or reduced without having to work in terms of electron-half-equations and electron transfers. You work out oxidation states by counting the numbers of electrons transferred. It would take far too long. Instead you learn some simple rules, and do some very simple sums! or S , or whether it has a giant structure like carbon or silicon. Note: It has been pointed out to me that there are a handful of obscure compounds of the elements sodium to caesium where the metal forms a negative ion - for example, Na - . That would give an oxidation state of -1. You can ignore these if you are doing chemistry at A level or its equivalent. The generalisation that Group 1 metals always have an oxidation state of +1 holds good for all the compounds you are likely to meet. If you are interested in these odd compounds, do an internet search for alkalides . The reasons for the exceptions Hydrogen in the metal hydrides Metal hydrides include compounds like sodium hydride, NaH. In this, the hydrogen is present as a hydride ion, H - . The oxidation state of a simple ion like hydride is equal to the charge on the ion - in this case, -1. Alternatively, you can think of it that the sum of the oxidation states in a neutral compound is zero. Since Group 1 metals always have an oxidation state of +1 in their compounds, it follows that the hydrogen must have an oxidation state of -1 (+1 -1 = 0). Oxygen in peroxides Peroxides include hydrogen peroxide, H 2 O 2 . This is an electrically neutral compound and so the sum of the oxidation states of the hydrogen and oxygen must be zero. Since each hydrogen has an oxidation state of +1, each oxygen must have an oxidation state of -1 to balance it. Oxygen in F 2 O The problem here is that oxygen isn't the most electronegative element. The fluorine is more electronegative and has an oxidation state of -1. In this case, the oxygen has an oxidation state of +2. Chlorine in compounds with fluorine or oxygen There are so many different oxidation states that chlorine can have in these, that it is safer to simply remember that the chlorine doesn't have an oxidation state of -1 in them, and work out its actual oxidation state when you need it. You will find an example of this below. Examples of working out oxidation states What is the oxidation state of chromium in Cr 2+ ? What is the oxidation state of chromium in CrCl 3 ? This is a neutral compound so the sum of the oxidation states is zero. Chlorine has an oxidation state of -1. If the oxidation state of chromium is n : n + 3(-1) = 0 n = +3 (Again, don't forget the + sign!) What is the oxidation state of chromium in Cr(H 2 O) 6 3+ ? This is an ion and so the sum of the oxidation states is equal to the charge on the ion. There is a short-cut for working out oxidation states in complex ions like this where the metal atom is surrounded by electrically neutral molecules like water or ammonia. What is the oxidation state of chromium in the dichromate ion, Cr 2 O 7 2- ? The oxidation state of the oxygen is -2, and the sum of the oxidation states is equal to the charge on the ion. Don't forget that there are 2 chromium atoms present. 2n + 7(-2) = -2 Warning: Because these are simple sums it is tempting to try to do them in your head. If it matters (like in an exam) write them down using as many steps as you need so that there is no chance of making careless mistakes. Your examiners aren't going to be impressed by your mental arithmetic - all they want is the right answer! If you want some more examples to practice on, you will find them in most text books, including my chemistry calculations book . What is the oxidation state of copper in CuSO 4 ? Unfortunately, it isn't always possible to work out oxidation states by a simple use of the rules above. The problem in this case is that the compound contains two elements (the copper and the sulphur) whose oxidation states can both change. The only way around this is to know some simple chemistry! There are two ways you might approach it. (There might be others as well, but I can't think of them at the moment!) You might recognise this as an ionic compound containing copper ions and sulphate ions, SO 4 2- . To make an electrically neutral compound, the copper must be present as a 2+ ion. The oxidation state is therefore +2. You might recognise the formula as being copper(II) sulphate. The "(II)" in the name tells you that the oxidation state is 2 (see below). You will know that it is +2 because you know that metals form positive ions, and the oxidation state will simply be the charge on the ion. Using oxidation states In naming compounds You will have come across names like iron(II) sulphate and iron(III) chloride. The (II) and (III) are the oxidation states of the iron in the two compounds: +2 and +3 respectively. That tells you that they contain Fe 2+ and Fe 3+ ions. This can also be extended to the negative ion. Iron(II) sulphate is FeSO 4 . There is also a compound FeSO 3 with the old name of iron(II) sulphite. The modern names reflect the oxidation states of the sulphur in the two compounds. The sulphate ion is SO 4 2- . The oxidation state of the sulphur is +6 (work it out!). The ion is more properly called the sulphate(VI) ion. The sulphite ion is SO 3 2- . The oxidation state of the sulphur is +4 (work that out as well!). This ion is more properly called the sulphate(IV) ion. The ate ending simply shows that the sulphur is in a negative ion. So FeSO 4 is properly called iron(II) sulphate(VI), and FeSO 3 is iron(II) sulphate(IV). In fact, because of the easy confusion between these names, the old names sulphate and sulphite are normally still used in introductory chemistry courses. Note: Even these aren't the full name! The oxygens in the negative ions should also be identified. FeSO 4 is properly called iron(II) tetraoxosulphate(VI). It all gets a bit out of hand for everyday use for common ions. Using oxidation states to identify what's been oxidised and what's been reduced This is easily the most common use of oxidation states. Oxidation involves an increase in oxidation state Reduction involves a decrease in oxidation state In each of the following examples, we have to decide whether the reaction involves redox, and if so what has been oxidised and what reduced. This is the reaction between magnesium and hydrochloric acid or hydrogen chloride gas: Mg + 2HCl MgCl 2 + H 2 Have the oxidation states of anything changed? Yes they have - you have two elements which are in compounds on one side of the equation and as uncombined elements on the other. Check all the oxidation states to be sure:.  The magnesium's oxidation state has increased - it has been oxidised. The hydrogen's oxidation state has fallen - it has been reduced. The chlorine is in the same oxidation state on both sides of the equation - it hasn't been oxidised or reduced. The reaction between sodium hydroxide and hydrochloric acid is: NaOH + HCl NaCl + H 2 O Checking all the oxidation states: Nothing has changed. This isn't a redox reaction. This is a sneaky one! The reaction between chlorine and cold dilute sodium hydroxide solution is: 2NaOH + Cl 2 NaCl + NaClO + H 2 O Obviously the chlorine has changed oxidation state because it has ended up in compounds starting from the original element. Checking all the oxidation states shows: The chlorine is the only thing to have changed oxidation state. Has it been oxidised or reduced? Yes! Both! One atom has been reduced because its oxidation state has fallen. The other has been oxidised. This is a good example of a disproportionation reaction. A disproportionation reaction is one in which a single substance is both oxidised and reduced. Using oxidation states to identify the oxidising and reducing agent This is just a minor addition to the last section. If you know what has been oxidised and what has been reduced, then you can easily work out what the oxidising agent and reducing agent are. This is the reaction between chromium(III) ions and zinc metal: 2Cr 3+ + Zn 2Cr 2+ + Zn 2+ The chromium has gone from the +3 to the +2 oxidation state, and so has been reduced. The zinc has gone from the zero oxidation state in the element to +2. It has been oxidised. So what is doing the reducing? It is the zinc - the zinc is giving electrons to the chromium (III) ions. So zinc is the reducing agent. Similarly, you can work out that the oxidising agent has to be the chromium(III) ions, because they are taking electrons from the zinc. This is the equation for the reaction between manganate(VII) ions and iron(II) ions under acidic conditions. This is worked out further down the page. Looking at it quickly, it is obvious that the iron(II) ions have been oxidised to iron(III) ions. They have each lost an electron, and their oxidation state has increased from +2 to +3. The hydrogen is still in its +1 oxidation state before and after the reaction, but the manganate(VII) ions have clearly changed. If you work out the oxidation state of the manganese, it has fallen from +7 to +2 - a reduction. So the iron(II) ions have been oxidised, and the manganate(VII) ions reduced. What has reduced the manganate(VII) ions - clearly it is the iron(II) ions. Iron is the only other thing that has a changed oxidation state. So the iron(II) ions are the reducing agent. Similarly, the manganate(VII) ions must be the oxidising agent. Using oxidation states to work out reacting proportions This is sometimes useful where you have to work out reacting proportions for use in titration reactions where you don't have enough information to work out the complete ionic equation. Remember that each time an oxidation state changes by one unit, one electron has been transferred. If one substance's oxidation state in a reaction falls by 2, that means that it has gained 2 electrons. Something else in the reaction must be losing those electrons. Any oxidation state fall by one substance must be accompanied by an equal oxidation state increase by something else. This example is based on information in an old AQA A' level question. Ions containing cerium in the +4 oxidation state are oxidising agents. (They are more complicated than just Ce 4+ .) They can oxidise ions containing molybdenum from the +2 to the +6 oxidation state (from Mo 2+ to MoO 4 2- ). In the process the cerium is reduced to the +3 oxidation state (Ce 3+ ). What are the reacting proportions? The oxidation state of the molybdenum is increasing by 4. That means that the oxidation state of the cerium must fall by 4 to compensate. But the oxidation state of the cerium in each of its ions only falls from +4 to +3 - a fall of 1. So there must obviously be 4 cerium ions involved for each molybdenum ion. The reacting proportions are 4 cerium-containing ions to 1 molybdenum ion. Or to take a more common example involving iron(II) ions and manganate(VII) ions . . . A solution of potassium manganate(VII), KMnO 4 , acidified with dilute sulphuric acid oxidises iron(II) ions to iron(III) ions. In the process, the manganate(VII) ions are reduced to manganese(II) ions. Use oxidation states to work out the equation for the reaction. The oxidation state of the manganese in the manganate(VII) ion is +7. The name tells you that, but work it out again just for the practice! In going to manganese(II) ions, the oxidation state of manganese has fallen by 5. Every iron(II) ion that reacts, increases its oxidation state by 1. That means that there must be five iron(II) ions reacting for every one manganate(VII) ion. The left-hand side of the equation will therefore be: MnO 4 - + 5Fe 2+ + ? The right-hand side will be: Mn 2+ + 5Fe 3+ + ? After that you will have to make guesses as to how to balance the remaining atoms and the charges. In this case, for example, it is quite likely that the oxygen will end up in water. That means that you need some hydrogen from somewhere. That isn't a problem because you have the reaction in acid solution, so the hydrogens could well come from hydrogen ions. Eventually, you will end up with this: Personally, I would much rather work out these equations from electron-half-equations! | If this is the first set of questions you have done, please read the before you start. You will need to use the BACK BUTTON on your browser to come back here afterwards. | Where would you like to go now? To the Redox menu . . . To the Inorganic Chemistry menu . . . To Main Menu . . . © Jim Clark 2002 (last modified November 2021)  Have an account? Suggestions for you See more 10th - University Redox reactions, 9th - 10th , naming ionic compounds, 10th - 12th , naming compounds, 10th - 11th , element symbols and names, 8th - 12th , moles and mole conversions, 9th - 12th , ionic bonding.  OXIDATION NUMBER QUIZ11th - 12th grade.  30 questions  Introducing new Paper modeNo student devices needed. Know more - 1. Multiple Choice Edit 1 minute 1 pt What is oxidation number of H in H 2 O? 0 -2 -1 +1
- 2. Multiple Choice Edit 45 seconds 1 pt What is oxidation number of Cr in Cr 2 O 7 2- ? -2 +2 +6 +12
- 3. Multiple Choice Edit 1 minute 1 pt What is oxidation number of P in K 3 PO 4 ? +1 +5 -2 0
- 4. Multiple Choice Edit 1.5 minutes 1 pt What is oxidation number of H in CaH 2 ? +1 -1 0 +2
- 5. Multiple Choice Edit 45 seconds 1 pt What is oxidation number of Fe in FeO ? +2 -2 0 +1
- 6. Multiple Choice Edit 1 minute 1 pt What is oxidation number of Ca in CaCl 2 ? -1 -2 +1 +2
- 7. Multiple Choice Edit 45 seconds 1 pt What is oxidation number of O in O 2 ? 0 -2 +1 +2
- 8. Multiple Choice Edit 1 minute 1 pt What is oxidation number of O in H 2 O 2 ? -2 -1/2 +1 -1
- 9. Multiple Choice Edit 1 minute 1 pt What is oxidation number of Na in Na + ? 0 +1/2 +1 -1
- 10. Multiple Choice Edit 20 seconds 1 pt What is oxidation number of Mn in MnO 2 ? 0 +2 -2 +4
- 11. Multiple Choice Edit 3 minutes 1 pt What is the charge for an ion in group 1? +1 -7 -1 +7
- 12. Multiple Choice Edit 3 minutes 1 pt What is the charge of an ion in group 2? +2 -6 -2 +6
- 13. Multiple Choice Edit 20 seconds 1 pt The sum of all oxidation numbers in a neutral compound is ___. 0 1 -1 depends on the compound
What is oxidation number of Hg in Hg 2 2+ ? What is the oxidation number of Magnesium (MgO)? whats an oxidation number What is the oxidation number for Flourine (F) in its compounds? What element always has an oxidation number of +1 when in combination with non metals? - 18. Multiple Choice Edit 30 seconds 1 pt Which of the following elements does NOT have an oxidation number of +2? Ca Sr K Ba
What is the oxidation number of Fe in Fe 3 O 4 ? What is the oxidation number of C in CO 2 ? - 21. Multiple Choice Edit 45 seconds 1 pt What is the oxidation number of N in NO 2 -1 ? -3 +4 -2 +3
What is the oxidation number of chlorine in HClO? What is the oxidation number of nitrogen in N 2 ? The sum of the oxidation numbers in SCN - is equivalent to The sum of the oxidation numbers in Na 2 CO 3 is equivalent to What is the oxidation number of iodine in KIO 3 ? Determine the oxidation number of chromium in CrO 4 2- . Determine the oxidation number of phosphorous in PO 2 - . What is the oxidation number of nickel in NiSO 3 ? - 30. Multiple Choice Edit 20 seconds 1 pt What is the oxidation of C in CH 4 ? -4 -1 +4 +1
Explore all questions with a free account  Continue with email Continue with phone  - Periodic table of the elements
- Chemistry calculators
- Image gallery
OXIDATION NUMBERS CALCULATORTo calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+ , HF2^- , Fe4[Fe(CN)6]3 , NH4NO3 , so42- , ch3cooh , cuso4*5h2o ). The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with the oxidation state. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Bonds between atoms of the same element (homonuclear bonds) are always divided equally. 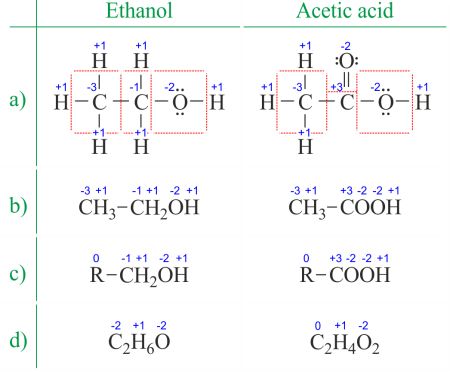 When dealing with organic compounds and formulas with multiple atoms of the same element, it's easier to work with molecular formulas and average oxidation numbers (Figure 1d). Organic compounds can be written in such a way that anything that doesn't change before the first C-C bond is replaced with the abbreviation R (Figure 1c). Unlike radicals in organic molecules, R cannot be hydrogen. Since the electrons between two carbon atoms are evenly spread, the R group does not change the oxidation number of the carbon atom it's attached to. You can find examples of usage on the Divide the redox reaction into two half-reactions page. Rules for assigning oxidation numbers- The oxidation number of a free element is always 0.
- The oxidation number of a monatomic ion equals the charge of the ion.
- Fluorine in compounds is always assigned an oxidation number of -1.
- The alkali metals (group I) always have an oxidation number of +1.
- The alkaline earth metals (group II) are always assigned an oxidation number of +2.
- Oxygen almost always has an oxidation number of -2, except in peroxides (H 2 O 2 ) where it is -1 and in compounds with fluorine (OF 2 ) where it is +2.
- Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals.
- The algebraic sum of the oxidation numbers of elements in a compound is zero.
- The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
Assigning oxidation numbers to organic compounds- cysteine: HO2CCH(NH2)CH2SH
 Back to top ↑ Citing this page: Generalic, Eni. "Oxidation numbers calculator." EniG. Periodic Table of the Elements . KTF-Split, 18 Jan. 2024. Web. {Date of access} . <https://www.periodni.com/oxidation_numbers_calculator.php>.  - Online calculators
- Scientific calculator
- Preparation of solutions
- Oxidation numbers calculator
- Balancing redox reactions
- Memory game
- Articles and tables
- Chemistry dictionary
- Printable periodic table
- Portable applications
- Chemistry images gallery
- Short form of the periodic table
- Long form of the periodic table
- History of the Periodic table of elements
- Electronic configurations of the elements
- Alphabetical list of chemical elements
- Naming of elements of atomic numbers greater than 100
- ASCII Periodic table
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Labeling of chemical containers
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Find the pairs
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Climate change
- Global warming and mankind
- Story of ozone and ozone holes
- World War 3: Battle for Earth
- The ozone layer is not a shield
- Hexadecimal color codes
- Writing equations on the Web
- Writing chemical equations on the Web
- Character entity references in HTML
- Unicode UTF-8 encoding
- Download PDF Documents
- Download Software
- Download Images
- Periodic table
 - Français
- Español
- Search on periodni.com
 | 









IMAGES
VIDEO
COMMENTS
O = -2. The oxidation number of oxygen in a compound is usually -2. If, however, the oxygen is in a class of compounds called peroxides (for example, hydrogen peroxide), then the oxygen has an oxidation number of -1. The sum of all oxidation numbers in a neutral compound is zero. NH4+. N = -3.
Study with Quizlet and memorize flashcards containing terms like 1. Free elements are assigned an oxidation number of 0., 2. The oxidation number for any simple monatomic anion or cation is equal to its charge., 3. Alkali metals (group 1A) in ionic compounds are always assigned a +1 oxidation number. and more.
5C. Al is +3. 6. the oxidation number of each hydrogen atom in a compound is +1, unless it is only combined with a metal atom, then it is -1. 7. the oxidation number of each oxygen atom in compounds is usually -2. When combined with fluorine atoms, oxygen becomes +2. In peroxides, an oxygen atom has an oxidation number of -1.
Oxygen's oxidation number is -2 except when in hydrogen peroxide (H 2 O 2), or a peroxide ion (O 2 2-) where it is -1. Hydrogen's oxidation number is +1, except for when bonded to metals as the hydride ion forming binary compounds. In LiH, NaH, and CaH 2, the oxidation number is -1. Fluorine has an oxidation number of -1 in all of its ...
Write the oxidation number with the sign of the charge followed by its value. For example, write +1 and -3 rather than 1+ and 3-. The latter form is used to indicate oxidation state. The oxidation number of a free element or neutral molecule is 0. For example, the oxidation number of C, Ne, O 3, N 2, and Cl 2 is 0.
Oxidation Number Rules. The convention is that the cation is written first in a formula, followed by the anion. For example, in NaH, the H is H-; in HCl, the H is H+. The oxidation number of a free element is always 0. The atoms in He and N 2, for example, have oxidation numbers of 0. The oxidation number of a monatomic ion equals the charge of ...
Oxidation numbers are assigned to elements using these rules: Rule 1: The oxidation number of an element in its free (uncombined) state is zero — for example, Al (s) or Zn (s). This is also true for elements found in nature as diatomic (two-atom) elements. Rule 3: The sum of all oxidation numbers in a neutral compound is zero.
Study with Quizlet and memorize flashcards containing terms like Step 1, Step 2, Step 3 and more.
The (II) and (III) are the oxidation states of the iron in the two compounds: +2 and +3 respectively. That tells you that they contain Fe 2+ and Fe 3+ ions. This can also be extended to the negative ion. Iron (II) sulphate is FeSO 4. There is also a compound FeSO 3 with the old name of iron (II) sulphite.
The School For Excellence 2019 ATAR Central - Chemistry Page 2 Part B: Identify the species being oxidised and reduced in each of the following reactions and write their
This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following som...
Oxidation number of O in the compound H 2 O: 0. -1. -2. +2. Element that only has a stable oxidation number of +1: Element that only has a stable oxidation number of +2: Element that only has a stable oxidation number of +3: What is the total oxidation number of elements in H.
The oxidation number of a monatomic ion is equal in magnitude and sign to its ionic charge. For example, the oxidation num- ber of the bromide ion (Br1 y) is y1; that of the Fe3àion is à3. 2. The oxidation number of hydrogen in a compound is à1, except in metal hydrides, such as NaH, where it is y1. 3.
What are the oxidation numbers of the atoms in this reaction? Check all that apply. CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g) The gain of electrons is called. reduction. The loss of electrons is called. oxidation. Use the drop-down menus to complete the sentences.
The correct rule for assigning oxidation numbers is: Hydrogen is usually +1, except when it is bonded to metals, where it is -1. Oxygen is usually -2, except in peroxides and when bonded to fluorine, where it is -1. A pure group 1 element is +1, and a pure group 2 element is +2. A monatomic ion's oxidation number is equal to its charge.
OXIDATION NUMBER QUIZ quiz for 11th grade students. Find other quizzes for Chemistry and more on Quizizz for free!
flourine. O.N. = -1. Oxygen. O.N. = -1 peroxides. O.N. = -2 in all other compounds aside from F. Group 17 (halogens) O.N. = -1 in combiniation with metals, nonmetals (except O), and other halogens lower in the group. Study with Quizlet and memorize flashcards containing terms like elemental form, monatomic ion, rule #3 and more.
Select all that apply. Check all that apply All elements in a neutral molecule have an oxidation number of zero. The sum of the oxidation numbers for the atoms in a neutral compound is zero. The oidation number for a monatomic n i the same as ts charge. If two atoms are bonded in a molecule, such as O2, the oxidation number for one atom is +1 ...
Start studying Assigning Oxidation numbers. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Rules for assigning oxidation numbers. The oxidation number of a free element is always 0. The oxidation number of a monatomic ion equals the charge of the ion. Fluorine in compounds is always assigned an oxidation number of -1. The alkali metals (group I) always have an oxidation number of +1. The alkaline earth metals (group II) are always ...